Unit of Heat - SI Unit, CGS Unit & Other Heat Units, FAQs
Introduction
Heat is a form of energy possessed by a body, also called thermal energy of the body. The S.I unit of heat is Joule (J) just like all other forms of energy. It flows from one body to another or from one system to another having different temperatures. Hence both heat and temperature are very closely related although their concepts are not the same. Temperature is measured in Kelvin which is the S.I unit. In fact Kelvin is also the C.G.S unit of temperature. Heat is measured in C.G.S unit also in addition to the S.I unit of heat energy. C.G.S unit of heat is also very commonly used. C.G.S unit of heat is Calorie (Cal). Another unit of heat is B.T.U and the B.T.U full form is British Thermal Unit. It is more of a traditional unit of heat which is still used in Britain as well as in U.S.A in some fields. Generally the symbol for heat is represented by Q. Another important term related to heat is heat capacity. The heat capacity unit is Joule per Kelvin. We will discuss heat capacity later in the article.
JEE Main/NEET 2027: Physics Important Formulas for Class 10
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs

How do we define heat and where does it come from?
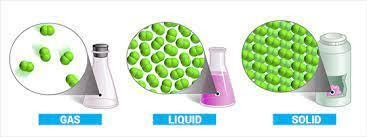
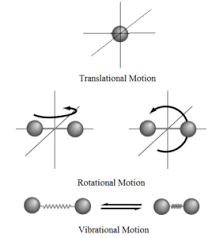
There are three states of matter- solids, liquids and gases, each of which are made up of molecules. These molecules possess kinetic energy due to their translational motion, vibrational motion and rotational motion (see fig 2).
 (Fig-1)
(Fig-1)
 (Fig-2)
(Fig-2)
The kinetic energy associated with these random motions of molecules and the configuration of these molecules with respect to each other (potential energy) is called the internal energy of the matter and the heat energy is part of this internal energy.
So we can define heat energy as a part of internal energy which flows between two bodies or systems when they have difference in temperature between them and it flows from higher temperature to the body at lower temperature.
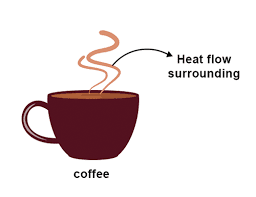
Examples- 1) When we put a cup of hot coffee in the table, it gets cold in a matter of time. This is because there is difference in temperature between the coffee and the air around it. Thus the heat flows from coffee to the atmosphere.
 (Fig-3)
(Fig-3)
2) When there is friction between two bodies heat energy is produced. For example rubbing our hands together. In this case the mechanical energy is transformed into heat energy.
 (Fig-4)
(Fig-4)
3) If we compress a gas, the molecules collide with each other more often which increases their internal energy (pressure is increased). Hence the gas gets hotter due to the heat energy produced. See the figure below.
 (Fig-5)
(Fig-5)
Thus heat energy either comes from internal energy of the body or is produced when any another form of energy like mechanical energy, chemical energy or electrical energy gets transformed into heat energy.
Also read -
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 12 Physics
- NCERT Solutions for All Subjects
Units of heat
S.I. unit of heat is Joules (J).James Prescott Joule found that when mechanical work (W) is converted to heat energy (Q), W by Q ratio comes out to be constant which is represented by unit joule.
i.e. W/Q=J
Dimensional formula for joules is (kgm2s-2) –> [ML2T-2].
C.G.S. unit of heat is Calorie (Cal).
1 Cal= 4.186 J
We can define 1 calorie of heat energy as the quantity of heat energy required to raise temperature of 1 gram of water by 1 ̊C.
However, in B.T.U. - 1 btu =1055.05585 J
How temperature and heat are closely related?
Temperature and heat is not the same thing although they are very closely related. In fact, the measurement of heat is called temperature. It measures the degree i.e. how cold or how hot the body is.
Example- When we have a high fever, we use thermometer that actually records how much our body is heated up, that is the temperature of the body.
 (Fig-6)
(Fig-6)
When any two bodies of different temperatures are in contact with each other, heat flows from the body at higher temperature to the body at lower temperature and this heat flow continues till their temperatures become equal. This is called temperature equilibrium. Thus we can define temperature equilibrium between two bodies as follows- two bodies are said to be in thermal equilibrium if no heat is transferred between them when they are in contact. Based on this concept of thermal equilibrium, an important law of thermodynamic (study of dynamic/flow of heat) called Zeroth law of thermodynamics is there which states that if 2 bodies A and B are in thermal equilibrium and B and C are in thermal equilibrium, then A and C are also in thermal equilibrium.
Note-Temperature is one of the seven fundamental quantities along with length, time, mass, ampere, mole and candela (used to measure luminous intensity).
Related Topics Link, |
Units of temperature
Generally, a thermometer is used to measure temperature. However different types of thermometers are there to be used in different situations. Other than thermometers, we also use thermocouples, infrared sensors, silicon diodes, change of state sensors etc. There are 2 fixed reference points while measuring temperature- freezing point of water and boiling point of water.
The different units of measurement are-
- Celsius scale ( ̊ C) - (Designed by Celsius) In this scale freezing point is 0 ̊ C and boiling point is 100 ̊C. Between these 2 points, there are 100 equal divisions.
- Fahrenheit scale ( ̊ F) - In this scale freezing point is 32 ̊ F and boiling point is 212 ̊ F.
Between these two points there are 180 divisions.
Relation between Celsius and Fahrenheit scale is- ̊ C = ( 5/9) ( ̊ F - 32) eq-(1)
- Kelvin scale (K) - This is the S.I. unit of thermodynamic temperature. At 0K it is absolute zero temperature. Therefore it is also called the absolute scale. On this scale the freezing point is 273.15K ~ 273K and the boiling point is 373K.
Relation between Celsius and Kelvin scale is – K = ̊ C + 273 eq-(2)
In both C.G.S. and M.K.S unit for temperature, we use Kelvin scale. See the figure below and compare the freezing and boiling points of water on different scales.
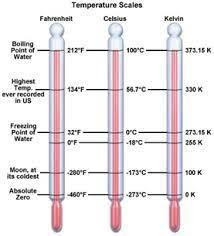 (Fig-7)
(Fig-7)
See the graph of pressure vs. temperature of a gas below. Upon extrapolating this graph it meets absolute zero at -273.15 ̊ C. At this point, the internal energy of the gas becomes zero. All molecular motions cease to exist and below this temperature, volume of the gas becomes negative which is impossible. Thus the lowest temperature one can reach is 0K. In fact, it is also practically not possible to reach absolute zero. The closest that scientists have reached to absolute zero is 150 Nano Kelvin but not 0K.
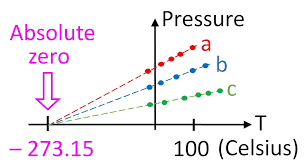
(Fig-7)
Remember - the S.I. unit of heat and temperature are J and kelvin respectively.
Also Read:
- NCERT solutions for Class 11 Physics Chapter 11 Thermal Properties of Matter
- NCERT Exemplar Class 11 Physics Solutions Chapter 11 Thermal Properties of Matter
- NCERT notes Class 11 Physics Chapter 11 Thermal Properties of Matter
Heat capacity
Heat capacity is the amount of heat required to raise temperature of a gas by 1 ̊ C. However we usually use the quantity ‘specific heat capacity’ that is the amount of heat required to raise temperature of a unit mass of the substance by 1 ̊ C. Hence heat capacity is an intensive thermodynamic quantity, that is, it depends on mass of the substance while specific heat capacity is an extensive thermodynamic quantity as it doesn’t depend on mass. Mathematically we can find the formula for specific heat as-
Experiments show that if ΔQ is a small amount of heat required to raise temperature of ‘m’ mass of a substance through temperature ΔT, then we can write
ΔQ ∝ m and ΔQ ∝ ΔT
- ΔQ ∝ mΔT
- ΔQ=C(mΔT)
- C=ΔQ/ ( mΔT), this is the formula for specific heat capacity.
Also check-
- NCERT Exemplar Class 11th Physics Solutions
- NCERT Exemplar Class 12th Physics Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Physics Notes:
Frequently Asked Questions (FAQs)
Using equation (1), we put x in place of the temperature in degree Fahrenheit and degree Celsius and solve for x. We will get -40 as our answer.
A calorimeter is a device used for measuring the amount of heat absorbed or evolved during any process. It can also measure heat capacity for us.
Molar heat capacity is the amount of heat required to be added to 1 mole of a substance to increase its temperature by 1 degree unit.
As there are 180 divisions in Fahrenheit scale and 100 divisions in Celsius scale and Kelvin scale. Thus 1 ̊F is smaller than the other two.
75.32J per mol per degree celsius
Also Read
02 Jul'25 07:03 PM
02 Jul'25 05:33 PM
02 Jul'25 05:08 PM
02 Jul'25 05:07 PM
02 Jul'25 05:04 PM
02 Jul'25 05:04 PM
02 Jul'25 05:00 PM
02 Jul'25 05:00 PM
02 Jul'25 05:00 PM
02 Jul'25 04:58 PM

