Phosphorous Acid (H3PO3) - Reactions, Structure, Properties, Uses, FAQs
Phosphorus acid (H3PO3) or phosphoric acid is an oxyacid of phosphorus. Phosphorous acid is also a conjugate acid of dihydrogen phosphite. This colorless compound is also given the name trihydroxy phosphorous acid since it contains three hydroxyl groups. Phosphorus acid chemical formula is H3PO3 or H3PO3 The name is phosphorus acid. Phosphorous acid, H3PO3 is a tribasic acid with one phosphorus, four oxygen, and three hydrogen atoms. And the basicity of phosphoric acid is 3 since it contains three acidic hydrogen atoms.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Preparation of Phosphorous Acid, H3PO3
- Toxicity of Phosphorous Acid, H3PO3
- Structure of H3PO3 or H3PO3 Structure
- Phosphorous Acid
- Reactions of Phosphorous Acid, H3PO3
- Redox Properties
- Basicity of Phosphoric Acid
- Uses of Phosphorous Acid, H3PO3.
- Phosphinic acid and Phosphonic Acid
Preparation of Phosphorous Acid, H3PO3
Phosphorous acid or H3PO3 is produced by the slow combustion reaction of phosphorus in the form of a white volatile powder. The salt of phosphorus acid is called phosphites. Phosphoric acid is prepared by the reaction of phosphorus trichloride that is PCl3 with water. It is also prepared by the reaction of tetra phosphorus hexoxide P4O6. Industrially it is prepared by spraying PCl3 into steam at 100 degrees Celsius, the reaction heat is then used for the removal of HCl and excess water vapor from it.
Toxicity of Phosphorous Acid, H3PO3
When phosphorous acid, H3PO3 contact with the eyes skin, and mucous membranes severely causes irritation and eye damage. It is also toxic when inhaled or by absorption by the skin. When a person inhales a particular amount of phosphorus acid it will cause irritation to the nose, throat, and lungs which will then lead to coughing, wheezing, and breathing problems. It severely damages the Respiratory system.
Phosphorus acid, H3PO3 is a white solid with sour order and is soluble in water. The taste of phosphorous acid eases garlic-like. From the chemical formula of phosphorus acid, we may think it is a tri-protic in nature that it ionizes two protons but actually it is diprotic. The molar mass of phosphorus acid is 81.9 g/mol with a density of 1.651g/cm3. The melting point of phosphorus acid eases 73.6 degrees Celsius and the boiling point of phosphorus acid is 200 degrees Celsius. It is soluble in water and also it is soluble in ethanol.
Also read -
| NCERT Solutions for Class 11 Chemistry |
| NCERT Solutions for Class 12 Chemistry |
| NCERT Solutions for All Subjects |
Structure of H3PO3 or H3PO3 Structure
The structure of H3PO3 can be easily explained in this portion. Phosphorus acid, H3PO3 contains one covalent bond and unity has the name trihydroxy ortho phosphorus acid suggesting that it contains three OH groups that are 3 hydrogen bond acceptors. The geometry of phosphorus acid is pseudo-tetrahedral. One oxygen forms a double bond with a Phosphorus atom, three OH groups are bonded directly with the phosphorus atom. The structure of phosphorus acid is shown below.
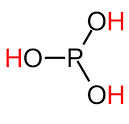
Phosphorous Acid
The structure of H3PO3 can be more accurately explained using the chemical formula HPO(OH)2. In the solid state phosphorous acid is tetrahedrally coordinated with one P-H and PO and two longer POH bonds. While it exists in equilibrium with the tautomer form containing three POH bonds. The one containing two hydroxyl groups is phosphonic acid while the other with three hydroxyl groups is phosphorous acid. The name ending with ‘ous’ means it is in the reduced form. The following structure shows this fact.
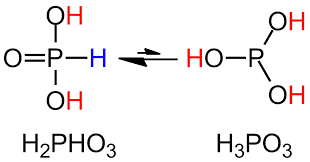
Tautomerism in phosphorous acid
Other important oxyacids are phosphoric acid and hypophosphorous acid. These compounds also show tautomerism similar to phosphorous acid by the shift in the position of H. This is all about the structure of H3PO3.
| Related topics |
Reactions of Phosphorous Acid, H3PO3
Acid property of H3PO3
Phosphorous acid, H3PO3 has a pKa value of 1.26-1.3. And it donates two hydrogen atoms only. The H atom which is bonded directly with the phosphorus is not readily ionized. The reaction can be represented as,
HPO(OH)2→HPO2(OH)-+H+
Thus one hydrogen ion is formed. The obtained product hydrogen phosphite ion is a weak acid with a pKa value of 6.7. It will also donate one hydrogen ion to form a phosphite ion. The reaction taking place is shown below.
HPO2(OH)-→HPO32-+H+
HPO32- is the second conjugate base of phosphorous acid with the name phosphite ion. This is the acidic property of phosphorous acid, the three hydrogens are not acidic
Redox Properties
Phosphorous acid, H3PO3 undergoes a disproportionation reaction at a temperature of 200 degrees Celsius to form phosphoric acid and phosphine.
4H3PO3→3H3PO4+PH3
This reaction is used for the preparation of phosphines that is PH3. Phosphorous acid slowly oxidizes in air to form phosphoric acid. The reaction of mercuric chloride with phosphorous acid forms mercurous chloride. While mercurous chloride reacts with phosphoric acid, H3PO3 to form mercury. Thus it shows redox properties.
Basicity of Phosphoric Acid
For phosphoric acid, H3PO3 three OH groups are bonded to the phosphorus atom so it is a tribasic group with a basicity of three. Basicity is the number of hydrogen atoms present in a chemical compound. Here the three hydrogen atoms are strongly bonded with an Electronegative atom of oxygen so it is acidic. While the basicity of phosphorus acid is only two. As all the hydrogen atoms are acidic it is dissociated completely in water except at low pH. When all three hydrogen ions have been removed the compound obtained is called orthophosphates. And the basicity of orthophosphoric acid is 2.
Uses of Phosphorous Acid, H3PO3.
Phosphorous acid, H3PO3 is widely used for the production of PVC stabilizers, amino methylene phosphonic acid, etc.
Because of its strong reducing nature, it can be used as a reducing agent.
It is used in the production of synthetic fibers and organophosphorus pesticides.
It is used for the production of amino trimethylene phosphonic acid which is used as a water treatment agent.
Phosphorous acid, H3PO3 is used to produce the fertilizer phosphate salt.
Phosphorous acid, H3PO3 also has many applications in the medical field mostly the dental field.
It is used for protecting ferrous materials including steel from rusting.
Basic lead phosphite is prepared by using phosphorous acid.
Phosphinic acid and Phosphonic Acid
The hypophosphorous acid known as phosphinic acid is also a phosphorous acid just like the phosphonic acid formula is H3PO3. The chemical formula of phosphinic acid is H3PO2 and it contains one phosphorus, three hydrogens, and only two oxygen atoms. It is a monoprotic compound in which only one hydrogen is acidic while for phosphonic acid it is diprotic with two acidic hydrogen atoms. The salt of hypophosphorous acid is hypophosphite. Like phosphonic acid, it also exists in equilibrium with the tautomer, HP(OH)2.
When phosphinic acid is heated it undergoes a disproportionation reaction to form phosphine and phosphoric acid. So it can be used for the synthesis of phosphoric acid. It is also used for reducing nickel. Its main application is the reduction of metal salts back to the bulk metal. Organo phosphinic acids can be easily synthesized using phosphinic acids and they have many important applications in various chemical reactions.
Also read :
Frequently Asked Questions (FAQs)
Formula of Phosphorous acid is H3PO3.
H3PO3 is an acid with the name phosphorous acid. It is a di protonic acid with many applications in many fields. Readily undergo ionization to form two hydrogen ions.
Phosphoric acid, H3PO3 is a colorless weak acid. For phosphoric acid the chemical formula is H3PO4. It contains one phosphorus atom, four oxygen atoms, and three hydrogen atoms. It is different from phosphorous acid, H3PO3 since it contains four oxygen atoms. For phosphoric acid, three OH groups are bonded to the phosphorus atom so it is a tribasic group with basicity of three. Basicity is the number of hydrogen atoms present in a chemical compound. And here the three hydrogen atoms are strongly bonded with an electronegative atom of oxygen so it is acidic. While the basicity of phosphorus acid is only two. As all the hydrogen atoms are acidic it is dissociated completely in water except at low pH. When all the three hydrogen ions have been removed the compound obtained is called orthophosphates.
Also Read
02 Jul'25 06:28 PM
02 Jul'25 06:27 PM
02 Jul'25 06:27 PM
02 Jul'25 06:13 PM
02 Jul'25 05:25 PM
02 Jul'25 05:21 PM
02 Jul'25 05:20 PM
02 Jul'25 04:56 PM
02 Jul'25 04:54 PM

