PCl5 Hybridization - Lewis Structure, Structure, Geometry FAQs
Have you ever thought about how a single atom can form bonds of equal strength and shape, even when its orbitals are different? The answer is hybridization. Hybridization is defined as the formation of a new degenerate orbital by mixing two atomic orbitals having the same energy. These hybrid orbitals have different shapes and energies from the original atomic orbitals. which is used to explain the bonding and geometries.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- PCl5 Hybridization
- $\mathrm{PCl}_5$ Structure
- $\mathrm{PCl}_5$ Lewis Structure
- Some Solved Examples
- Summary
In this article, we will cover the concept of Hybridization. This concept falls under the broader category of Chemical Bonding, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more.
PCl5 Hybridization
Compound
-
The energy of 3d ~ energy of 3s ~ energy of 3p, as well as the energy of 3d ~ energy of 4s ~ energy of 4p.
Because of the above reason, hybridization of 3rd-period elements includes 3d, 3p, and 3s or 3d, 4s, and 4p ( as the energy of s and p is equivalent to d) and there is also an energy difference between 3p and 4s orbital, which led to no hybridization of an element with 3p, 3d and 4s.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
The hybridization of
The molecular geometry of
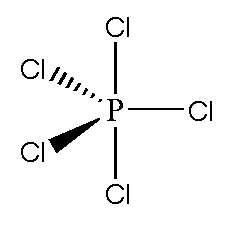
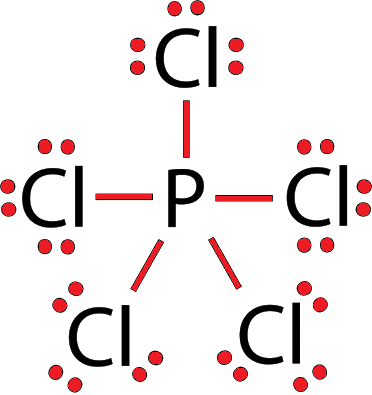
Also read :
- NCERT notes Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
- NCERT solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 4 Chemical Bonding and Molecular Structure
Some of our important hybridization, including s, p, and d orbital, are given below:-
|
Shape of molecules |
Types of hybridization |
Atomic orbitals |
Example |
|
|
dsp2 |
d+s+p(2) |
[Ni(CN)4]2– |
|
|
sp3d |
s+p(3)+d | |
|
|
sp3d |
s+p(3)+d |
BrF5 |
|
|
Sp3d2, d2sp3 |
s+p(3)+d(2), d(2)+s+p(3) |
[CrF6]3–, [Co(NH3)6]3+ |
Some Solved Examples
Q-1 Is
Ans:
Q-2 Explain the hybridization involved in phosphorus pentachloride.
Ans: The hybridization involved in P of
Q-3 Explain the formation of
Ans: Here we know that outermost electrons or valence electrons are 5 in the case of phosphorus, and the orbits are 1s, 3p, and 1d i..e.., available for hybridization. And the hybridization of
As we know trigonal bipyramidal is a closed figure so there must be bond angles we can discuss and the amazing fact is that not all the bond angles in
Q-4 PCl₅ shape according to VSEPR theory?
Ans: According to VSEPR theory shape of
Q-5 How many types of bonds are formed in
Ans: Types of a bond will be 2. There are two types of bonds that will be formed during molecule formation, i.e.,
Q-6 What are the bond angles of both equatorial bonds and axial bonds?
Ans: • Equatorial bonds, i.e, all 3 p – cl bonds which lie in the same plane. They are arranged by making an angle of 120°.
• Axial bond i.. i.e... all 2 p–Cl bonds in which one p – Cl bond lies above the equatorial surface and the other one p – Cl bond lies below the equatorial plane. These both p – cl bonds make an angle of 90° with p – cl bonds situated in an equatorial plane or equatorial bond. The angle between both p–cl bonds in axial position is 180° as they are situated just opposite to each other
Q-7 Which bond is weaker, equatorial or axial?
Due to repulsive interaction caused by the equatorial bond pair towards the axial bonds, the axial bond pairs are slightly longer to reduce repulsive interaction, but due to increasing its length its strength reduces ( i..e.. length of bond is inversely proportional to the strength of bond ) and so axial bonds are slightly weaker than equatorial bond due to more repulsive interaction from equatorial bond pairs.
Q-8 Hybridization of
- In a gaseous state, phosphorus of both
- But in solid state, p of
Q-9 What is the geometry of PCl4+?
- In
- So the geometry of
Practice More Questions With the Link Given Below:
Read useful topics:
Summary
NCERT Chemistry Notes :
Frequently Asked Questions (FAQs)
The hybridization of PCl5 (phosphorus pentachloride) is sp3d. This means that one s orbital, three p orbitals, and one d orbital of the phosphorus atom combine to form five equivalent hybrid orbitals, which are used to form bonds with the five chlorine atoms.
PCl5 uses sp3d hybridization because phosphorus needs to accommodate five bonding pairs around it. The sp3 hybridization only provides four hybrid orbitals, which is insufficient for PCl5. By including a d orbital in the hybridization, phosphorus can form the required five bonds.
The Lewis structure of PCl5 shows a central phosphorus atom bonded to five chlorine atoms. There are five single bonds between P and Cl, and no lone pairs on the phosphorus. Each chlorine atom has three lone pairs.
In
The molecular geometry of
The oxidation state of phosphorus in
The bond angle between the equatorial chlorine atoms in
The bond angle between an axial and an equatorial chlorine in
No, not all P-Cl bonds in
The hybrid orbitals in
VSEPR (Valence Shell Electron Pair Repulsion) theory explains that the five electron pairs around the phosphorus in
Yes,
In the gas phase,
The hybridization of PCl5 and PF5 is the same: both use sp3d hybridization. This is because both molecules have a central phosphorus atom bonded to five halogen atoms, resulting in the same trigonal bipyramidal geometry.
PCl5 is unstable at room temperature because it readily dissociates into PCl3 and Cl2. This is due to the weakness of the axial P-Cl bonds and the stability of the PCl3 molecule, which follows the octet rule.
The electronegativity difference between P and Cl makes the P-Cl bonds polar, with chlorine being more electronegative. This polarity contributes to the reactivity of PCl5, particularly its susceptibility to hydrolysis.
The chlorine atoms in PCl5 are not hybridized. They use their unhybridized p orbitals to form sigma bonds with the sp3d hybrid orbitals of phosphorus. Each chlorine atom also has three lone pairs in its other p orbitals.
The structure of PCl5, with its expanded octet and polar P-Cl bonds, contributes to its high reactivity. The trigonal bipyramidal geometry results in weaker axial bonds, making PCl5 prone to dissociation and nucleophilic attack.
The formal charge on the phosphorus atom in PCl5 is 0. This can be calculated as: [# of valence electrons (5)] - [# of non-bonding electrons (0)] - [1/2 * # of bonding electrons (10)] = 5 - 0 - 5 = 0.
PCl5 demonstrates hypervalency because the central phosphorus atom has more than eight electrons in its valence shell. This is possible due to the involvement of d orbitals in bonding, allowing phosphorus to expand its octet and form five bonds.
PCl5 can exhibit geometric isomerism in its reactions. For example, when it forms complexes like [PCl5F]-, there can be isomers where the F- ion is in either an axial or equatorial position relative to the trigonal bipyramidal PCl5 structure.
The P-Cl bond order in PCl5 is lower than in PCl3. In PCl5, the five bonds share the electron density from phosphorus, resulting in weaker individual bonds. In PCl3, there are only three bonds sharing the electron density, making them stronger.
Despite having polar P-Cl bonds, PCl5 has a net dipole moment of zero. This is because the trigonal bipyramidal structure results in a symmetrical distribution of charge, with the individual bond dipoles canceling each other out.
The larger atomic radius of phosphorus, compared to elements in the second period, allows it to accommodate five chlorine atoms around it. This larger size, along with the availability of d orbitals, enables phosphorus to expand its octet and form PCl5.
In PCl5, one d orbital of phosphorus participates in sp3d hybridization. This d orbital combines with one s and three p orbitals to form five equivalent hybrid orbitals, allowing phosphorus to form five bonds and expand its octet.
In PCl5, the electron domain geometry and molecular geometry are the same: trigonal bipyramidal. This is because all electron domains around phosphorus are bonding pairs, with no lone pairs. Therefore, the arrangement of atoms (molecular geometry) directly reflects the arrangement of electron domains.
The bond angle between the two axial chlorine atoms in PCl5 is 180°. This is because they are positioned directly opposite each other, above and below the plane of the equatorial chlorine atoms in the trigonal bipyramidal structure.
The presence of accessible d orbitals in phosphorus allows it to undergo sp3d hybridization. This hybridization produces five equivalent orbitals, enabling phosphorus to form five bonds and expand its octet, which is necessary for the formation of PCl5.
The structure of PCl5 influences its melting and boiling points through intermolecular forces. Despite being a polar molecule, PCl5 has relatively low melting and boiling points due to weak van der Waals forces between molecules, a result of its symmetrical charge distribution.
In the [PCl6]- ion, the phosphorus atom undergoes sp3d2 hybridization. This allows it to form six equivalent bonds with the chlorine atoms, resulting in an octahedral geometry.
In PCl5, the sp3d hybrid orbitals of phosphorus overlap with the p orbitals of chlorine to form sigma bonds. The overlap occurs end-on, resulting in strong covalent bonds between phosphorus and chlorine atoms.
The trigonal bipyramidal structure of PCl5, resulting from sp3d hybridization, leaves room for the phosphorus atom to accept another electron pair. This ability to expand its coordination number makes PCl5 a good Lewis acid, capable of forming adducts with Lewis bases.
The high electronegativity of chlorine makes the P-Cl bonds polar, with a partial negative charge on chlorine. This polarity contributes to the reactivity of PCl5, making it susceptible to nucleophilic attack and hydrolysis, which affects its stability.
The axial and equatorial positions in PCl5 are significant because they are not equivalent. The axial bonds are slightly longer and weaker than the equatorial bonds due to greater repulsion. This difference affects the reactivity and properties of PCl5.
Molecular orbital theory describes the bonding in PCl5 as a combination of atomic orbitals to form molecular orbitals. The sp3d hybrid orbitals of phosphorus combine with p orbitals of chlorine to form bonding and antibonding molecular orbitals, determining the overall stability of the molecule.
The structure of PCl5, with its symmetrical charge distribution, results in weak intermolecular forces. This leads to a relatively high vapor pressure, as less energy is required to overcome these forces and convert the substance from liquid to gas.
Resonance does not significantly apply to PCl5 in its ground state. The molecule is best described by a single Lewis structure with five single bonds. However, in reactions or excited states, resonance structures involving multiple bonds might be considered.
The 90° and 120° bond angles in PCl5 are a result of its trigonal bipyramidal geometry. The 120° angles between equatorial chlorines minimize repulsion in the equatorial plane, while the 90° angles between axial and equatorial chlorines allow for the most efficient packing of five atoms around the central phosphorus.
The trigonal bipyramidal structure of PCl5 makes it prone to substitution reactions, particularly at the axial positions. The axial bonds are longer and weaker, making them more susceptible to nucleophilic attack. This structural feature contributes to PCl5's usefulness as a chlorinating agent in organic synthesis.
The sp3d hybridization of PCl5 results in all electrons being paired, making the molecule diamagnetic. This means PCl5 is slightly repelled by magnetic fields, a property directly related to its electronic structure and hybridization.
Electron-domain repulsion theory (VSEPR) explains that the five bonding pairs of electrons in PCl5 arrange themselves to minimize repulsion. This results in the trigonal bipyramidal geometry, where the electron domains (bonds) are as far apart as possible, leading to the observed 90° and 120° bond angles.
The expanded octet in PCl5 allows phosphorus to form five bonds, exceeding the usual octet of eight electrons. This is possible due to the availability of d orbitals in phosphorus, demonstrating that the octet rule is not universal and can be violated by elements in the third period and beyond.
Despite having polar P-Cl bonds, PCl5 is overall non-polar due to its symmetrical structure. This makes it more soluble in non-polar solvents. However, its reactivity with polar solvents like water (resulting in hydrolysis) complicates its solubility behavior in polar media.
In its pure form, PCl5 does not conduct electricity as it does not have free ions or electrons. However, when dissolved in a polar solvent or melted, it can conduct electricity due to the formation of ions through
Also Read
12 Aug'25 08:43 AM
10 Aug'25 12:24 PM
04 Aug'25 06:34 PM
02 Jul'25 07:58 PM
02 Jul'25 07:57 PM
02 Jul'25 07:57 PM
02 Jul'25 07:55 PM
02 Jul'25 06:20 PM
02 Jul'25 06:20 PM
02 Jul'25 06:20 PM

