Nitrogen Dioxide - Preparation, Sources, Structure, Properties, Uses, FAQs
Nitrogen dioxide is a chemical compound and nitrogen oxide formula is represented by ![]() as the name as well as formula define the name that one nitrogen is present ad two atoms of oxygen are present which are termed as dioxide where di corresponds to two and the name is known as nitrogen dioxide.
as the name as well as formula define the name that one nitrogen is present ad two atoms of oxygen are present which are termed as dioxide where di corresponds to two and the name is known as nitrogen dioxide.
Nitrogen dioxide is also known by the name nitrogen (IV) oxide and Deutoxide of nitrogen where deuto also corresponds to two and oxide for oxygen atom. ![]() is said to be highly poisonous in nature and it is also known as the major pollutants present in atmosphere which absorb UV light i.e. ultra violet light and it helps them to stop to reach on the surface of the earth.
is said to be highly poisonous in nature and it is also known as the major pollutants present in atmosphere which absorb UV light i.e. ultra violet light and it helps them to stop to reach on the surface of the earth. ![]() chemical name is nitrogen dioxide.
chemical name is nitrogen dioxide.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Main Sources of Nitrogen Dioxide
- Preparation of Nitrogen Dioxide
- Uses of Nitrogen Dioxide
- Chemical properties of nitrogen dioxide:
- Adverse Effect of Nitrogen Dioxide
Nitrogen dioxide represented by ![]() color is yellowish brown if present in liquid form and reddish brown in color if it forms in gaseous state. The one main advantage of nitrogen dioxide when it is present in vapor form is that it is heavier in its vapor dorm as compare to air.
color is yellowish brown if present in liquid form and reddish brown in color if it forms in gaseous state. The one main advantage of nitrogen dioxide when it is present in vapor form is that it is heavier in its vapor dorm as compare to air.
![]() structure
structure
The structure of nitrogen dioxide can be represented as follows:
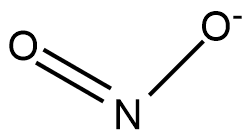
Nitrogen dioxide is one of the known or main compounds of nitrogen atoms. Molecular weight or molar mass of nitrogen dioxide is given by 46.006 g/mol whose density is 1.880 ![]() and boiling point or melting point of nitrogen dioxide is given as
and boiling point or melting point of nitrogen dioxide is given as ![]() and
and ![]() respectively. It is paramagnetic in nature where paramagnetic are those compounds which have unpaired electrons and impart color in ultra violet region.
respectively. It is paramagnetic in nature where paramagnetic are those compounds which have unpaired electrons and impart color in ultra violet region.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Main Sources of Nitrogen Dioxide
The primary source of nitrogen dioxide is nitric oxide represented and nitric oxide formula is given by NO i.e. one nitrogen and one oxygen atom. Around 98% of nitric oxide is emitted by the process called combustion where combustion is defined as the process in which any substance get burned in the presence of oxygen and produce heat and light during this process other than this combustion nitrogen is also emitted in the form of nitrogen oxide which is said to be somewhat non-toxic in nature but when it reacts with oxygen present in atmosphere then it rapidly got converted into nitrogen dioxide.
Nitrogen dioxide is said to be very much dangerous for human and effect respiratory functions of humans which increase respiratory disease in humans and increase pollutants in air. Nitrogen dioxide is also said as a precursor which is used in the formation of nitrate aerosols and nitrosamines and the adverse effect of these compounds on health effects are in continual study. On the basis of this study its adverse effects on public health and welfare are discussed in which nitrogen oxides are known as the pollutants present in atmosphere and for these types of standards and regularly controls have been given by the U.S. c.
Preparation of Nitrogen Dioxide
The one of most basic source of nitrogen dioxide is by the oxidation process of nitric oxide and the reaction can be shown as:
![]()
Nitric oxide is prepared when nitrogen and oxygen combine with each other and the reaction for the preparation of nitric oxide can be represented as following manner:
![]()
In the laboratory, nitrogen dioxide is prepared in two steps and in the first step dehydration process is takes place in this process nitric acid gives nitric acid and dinitrogen pentaoxide and the reaction can be shown as:
![]()
After this dinitrogen pentaoxide goes through thermal decomposition which can be shown as:
![]()
Nitrogen dioxide also undergoes thermal decomposition through some metals and this can be represented by the following reaction:
![]()
Other method is reduction of concentrated nitric acid by introducing itself by metal and for this reaction can be shown as:
![]()
It can also be prepared by adding nitric acid with tin and the reaction can be shown as:
![]()
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 7 The P-block elements
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 7 The P-block elements
- NCERT notes Class 12 Chemistry Chapter 7 The P-block elements
Uses of Nitrogen Dioxide
There are various application of nitrogen dioxide which can be explained as follows:
1. One of the basic application of nitrogen dioxide is it is used as an intermediate while producing nitric acid.
2. It is used in the manufacturing of oxidized cellulose compound.
3. The main application of nitrogen dioxide is it is used as catalyst.’
4. It is also used as an intermediate for producing sulphuric acid.
5. Nitrogen dioxide is also used as an oxidizer in case of rocket fuels.
6. It can uses as a nitrating agent.
7. It can be also used for bleaching of flour.
8. It can also be used as an oxidizing agent.
9. Nitrogen dioxide is also used as explosive agent.
| Related topics link, |
Chemical properties of nitrogen dioxide:
These properties can be explained as follows:
1. Thermal nature: Thermal nature of nitrogen dioxide is generally exists in equilibrium which produces dinitrogen tetroxide gas and the equilibrium constant for the reaction ![]() can be shown as:
can be shown as:
![]() .
.
2. Oxidizer: Nitrogen dioxide is act as a strong oxidizer due to the weakness of N-O bond.
3. Hydrolysis reaction: Hydrolysis reaction is the reaction of addition water molecules and the reaction can be shown as:
![]()
4. Nitrites formation: Nitrites are formed with the help of alkyl and metal iodide which can be represented as:
![]()
Another Reaction Can be Shown as:
5. At low concentrations of nitrogen dioxide it is negligible slow reaction.
Adverse Effect of Nitrogen Dioxide
There are many adverse effects of nitrogen dioxide on environment as well as on human beings this is noticed that nitrogen dioxide is of fatal nature. Nitrogen dioxide when comes in contact with eyes or skin it causes burning sensation. It forms frostbite when these are present in liquid form when nitrogen dioxide reacts with blood it forms methemoglobin. When nitrogen dioxide heat further it get decompose and release gas of nitric oxides which is toxic in nature. Nitrogen dioxide is also termed as irritated gas which cause inflammation at high concentration in airways.
Nitrogen dioxide mainly effects respiratory conditions and long term inhalation of nitrogen dioxide has many harmful effects on our health out of which some can be described as decrease the lung capacity, increase the possibility of respiratory diseases and also increase allergy from dust as it contains pollutants. It also produces small dust particles and ozone at ground level which put many adverse effect on our environment and also on our environment.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Nitric oxide and nitrogen dioxide both are known as the two most toxic and dangerous forms of nitrogen oxides. One of the most common form of oxides of nitrogen is nitrous oxide which is termed as laughing gas also known as greenhouse gas which helps in global warming.
The chemical name of NO is nitric oxide where nitric corresponds to nitrogen atom and oxide represents single atom of oxygen.
It is used as a catalyst in many chemical reactions, used as a fuel in rockets, used as an oxidizing agent and also for bleaching purposes.
Also Read
02 Jul'25 06:28 PM
02 Jul'25 06:27 PM
02 Jul'25 06:27 PM
02 Jul'25 06:13 PM
02 Jul'25 05:25 PM
02 Jul'25 05:21 PM
02 Jul'25 05:20 PM
02 Jul'25 04:56 PM
02 Jul'25 04:54 PM

