Huckel Rule - Definition, Explanation, Importance, FAQs
Huckel Rule or Define Huckel Rule
To check whether a closed molecule has aromatic properties Huckel rule is used. This role also has the name Huckel’s rule of aromaticity. This rule was put forward by the physical chemist Erich Huckel in 1931. This rule is also called the 4n+2 rule or 4n 2 rule. According to this rule when a cyclic molecule obeys 4n+2 pi electrons it is aromatic. Where n is the non-negative integer that is n=0, 1, 2,3… it is that much necessary to find the aromaticity as aromatic compounds have high stability. The aromatic compounds have higher stability it is due to the reason that aromatic compounds have the presence of the delocalized pi electrons that is they have a cyclic resonance in their skeleton. Let us take an example of benzene the structure of benzene is shown below.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Huckel Rule or Define Huckel Rule
- Huckel Rule of Aromaticity
- Cyclopentadiene
- Importance of Aromatic Compounds
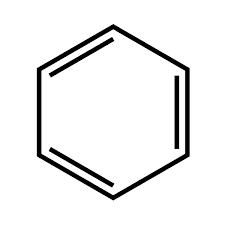
Structure of benzene
From the structure of benzene, we got that it contains three alternate double bonds. And all the carbon atom present in them is SP2 hybridized. So a cyclic resonance and delocalization of electrons are possible. Therefore it is a planar and cyclic compound. Benzene contains 6 pi electrons that is 4×1+2=6 which means that the value of n in the benzene compound is 1 which is an integer value so it is an aromatic compound. In addition to these rules, certain other criteria also should be avoided in order to be aromatic compounds. Those rules are described below.
The molecule should be cyclic that is a cyclic compound only possess aromatic property.
The molecule should be planar. For a molecule to be planar its hybridization must be SP2 or SP. When the hybridization is SP3 the planarity of a molecule is lost and it cannot be kept in the aromatic category. It also means that the orbitals present in the molecule should be parallel to participate in conjugation.
The molecule must contain wake and p orbitals that are there should not be any atom with the hybridization SP3.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Huckel Rule of Aromaticity
Explain huckel rule or Huckel theory.
For a cyclic molecule to be aromatic, it should follow the formula for the 4n+2 pi-electron rule. The huckel rule formula is given by: 4n+2. Where n is the integer with a positive value including zero. Aromaticity of benzene and the higher stability of benzene is established with the help of his Huckel rule. The value of n in the benzene compound is 1. Some of the compounds that obey this rule and are showing extra stability as pyrrole, pyridine, and furan. And all three compounds have a pi-electron number of 6.
Stability of monocyclic hydrocarbons
With the help of the Huckel rule stability of several monocyclic hydrocarbons can be well understood. The best example of a monocyclic hydrocarbon is benzene. Benzene is a cyclic compound with all the carbon atoms in the hybridization SP2 and it follows the Huckel rule of aromaticity that is the 4n+ 2 rule and it contains 6 pi electrons. So according to the rule of aromaticity benzene is a compound with aromatic properties and high stability as it follows all the criteria needed for an aromatic compound.
This is the reason behind benzene not participating in any additional reaction. Benzene only takes part in substitution reactions with the same number of double bonds or electrons after the reaction or in the product. This extra stability of benzene is called aromaticity. That means benzene is not ready to lose its high stability, every molecule tries to be in its most stable form. Other than benzene several other compounds also follow this extra stability. Let us give an example of furan. The structure of the furan is shown below.
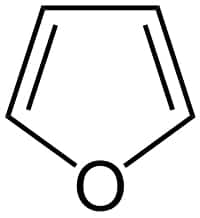
Structure of furan
From the structure of furan, we got the information that it contains two alternate double bonds with all the carbon atoms in the SP2 hybridization. So the delocalization of electrons is possible through the vacant P orbitals. Now calculating the number of pi electrons in furan it contains 6 pi electrons. Since the two double bonds are visible from the structure itself. Oxygen contains two lone pair electrons among which one lone pair electron participates in hybridization and is calculated as the pi electrons. Thus for furan, there are 6 pi electrons. And is an aromatic compound.
Following is the structure of the pyrrole.
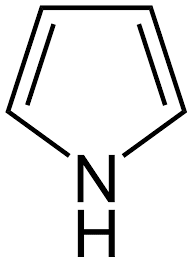
Structure of pyrrole
From the structure of pyrrole, it contains two alternate double bonds. The aromaticity of pyrrole can be explained just similarly to furan. Here the nitrogen atom is present and it contains one lone pair of electrons and is used for the delocalization of electrons. Therefore a cyclic resonance is developed and the molecule is planar since all the orbitals are parallel to each other. The number of pi electrons in pyrrole is 6 therefore the value of n is one. And the compound is aromatic. Considering the compound cyclopentadiene by using the Huckel rule of aromaticity we can easily predict the stability of its cation and anion also.
| Related topics link, |
Cyclopentadiene
The structure of cyclopentadiene is shown below.
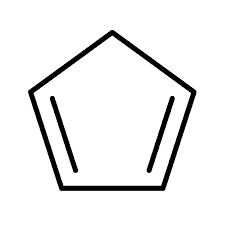
Structure of cyclopentadiene
From the structure of cyclopentadiene, the pi-electron count is 4, therefore the compound is not aromatic since it does not obey Huckel’s 4n+2 rule. For cyclopentadienyl anion, it has 6 pi electrons and is also quite stable. The structure of the cyclopentadienyl anion is shown below.
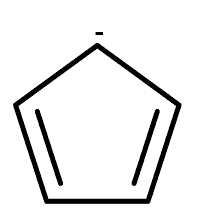
Structure cyclopentadienyl anion
From the structure itself, we got that it contains 6 pi-electrons by counting the negative charge on it. The compound is aromatic since it obeys Huckel’s 4n+2 rule. Cyclopentadiene contains only 4 pi electrons while cyclopentadienyl anion contains 6 pi electrons with the addition of one negative charge therefore it is an aromatic compound. Let us consider the cation of cyclopentadiene. The structure of the cyclopentadienyl cation is shown below.
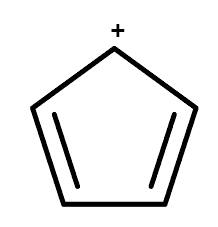
Structure of cyclopentadienyl cation
For the cation of cyclopentadiene, there are only 4 pi electrons. It is similar to cyclopentadiene’s case. So it is a multiple of 4 which means that it is an antiaromatic compound.
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT Exemplar Class 11 Chemistry Solutions Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT notes Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and techniques
Importance of Aromatic Compounds
Aromatic compounds have many e applications in industrial as well as in living things. The 20 basic building blocks of proteins that are histidine, phenylalanine, tryptophan, and tyrosine amino acids are aromatic compounds. Adenine, thymine, cytosine, guanine, and uracil are the five nucleotides that make the genetic code of DNA and RNA are all aromatic compounds that are purine or pyrimidines.
The aromatic compounds like benzene, toluene, ortho-xylene, and para xylenes are very important in industrial applications as well. The heterocyclic aromatic compounds like pyridine, pyrazine, pyrrole, imidazole, pyrazole, oxazole, thiophene, etc. are all very important. There are certain other fused aromatic compounds also that contain two or more rings fused. Naphthalene, anthracene, and phenanthrene are all very important.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
The rule that is used to check the aromaticity of a compound is the Huckel rule. According to this rule when a cyclic, planar, compound contains 4n+2 pi electrons the compound will be aromatic and stable. Benzene is a very common example with 6 pi electrons.
Cyclopentadienyl anion is aromatic. Since it contains 6 pi electrons this compound is aromatic.
Cyclopentadiene is not aromatic since the total number of pi electrons is only 4. And it does not obey the 4n+2 rule.
Pyrrole is aromatic since it contains 6 pi electrons it will obey Huckel's 4n+2 rule
The n value ranges in between 0 and 6
It is the experimental rule to predict the multiplicity in the NMR spectra.
Also Read
02 Jul'25 05:11 PM
02 Jul'25 05:10 PM
02 Jul'25 05:10 PM
02 Jul'25 05:05 PM
02 Jul'25 04:57 PM
02 Jul'25 04:57 PM
02 Jul'25 04:56 PM
02 Jul'25 04:56 PM
02 Jul'25 04:55 PM

