Difference Between Ideal Gas and Real Gas with FAQs
In this article, we will discuss the difference between real gas and ideal gas, non ideal gas, ideal and real gas, ideal and real gas examples.
Differentiate between ideal gas and real gas:
Ideal Gas | Real gas |
| Ideal gas has no definite volume | Real gas has definite volume |
| There are elastic collisions of particles in ideal gas. | There is non - elastic collisions between particles in non-ideal gas. |
| There is absence of intermolecular attraction force in ideal gas. | There is presence of intermolecular attraction force in real gas or non-ideal gas. |
| It does not exist in reality that is in the environment and is a hypothetical gas. | It exists in the environment |
| The pressure is high | The pressure is less when compared to other ideal /perfect gas. |
| It is independent | It interacts with other gases. |
| It obeys gas laws such as pV=nRT | It obeys p + ((n2 a)/V2)(V - nb) = nRT |
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
The above table gives the difference between real gas and ideal gas. So, we have assumed that all gases follow the gas laws under all conditions of temperature and pressure, however, this is not true for real gas. Difference between real gases and ideal gasis that real gas obeys the gas laws under limited conditions of low pressure and high temperature.
Theideal gas and real gas have major difference thatreal gases exhibit deviations from gaseous laws and the deviations increase when temperature and pressure are near to conditions in which the gas can condense into liquid, thus the Boyle’s and Charles’s law derived ideal gas equation is only applicable at relatively low pressure and moderately high temperatures.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Ideal gas and real gas difference are that ideal gas is a hypothetical gas that obeys the gas law exactly under all conditions of temperature and pressure which is perfect gas or ideal gas. The universal gas constant R, is an experimentally derived quantity which has the same value for all ideal gases.
Now, as we know that we can distinguish between ideal gas and real gas is that ideal gas equation pV= nRT, is not applicable to real gas, hence the evaluation of universal gas constant R cannot be done directly by using pressure, volume, and temperature data of real gas.
In ideal gas and real gas, we know through various experiments it is known that gases approach ideal behaviour as the pressure is decreased. Thus, the extrapolation method (p → 0) on the data of real gases can be utilized to determine the corresponding properties of ideal gas and hence the data gained from this method, after extrapolation, should be independent of the characteristic of the real gas used for experiment.
Here, ideal and real gas can be differentiated after calculating the volumes of one mole of a real gas at different pressures and constant temperature, a graph between pV and p can be drawn, then on extrapolating the graph to zero pressure and for departure from ideal behaviour it is now possible to determine the value of pV which is expected to be appropriate to one mole of ideal gas. By following this it is found that the value of (pV)p→0 at 273.15 K is 22.711 dm3 bar. Thus, if p=1 bar, then V=22.711 dm3. The volume occupied by one mole of an ideal gas at standard temperature 273.15 K and 1 bar pressure is 22.711 dm3.
In ideal gas and real gas, the difference between real and ideal gas is that real gas is the gases which do not obey the ideal gas laws exactly under all conditions of temperature and pressure and is also called as non-ideal gas. According to experiments at low pressure and moderately high temperature, the gases obey the laws of Boyle’s Charles and Avogadro approximately. But as the pressure rises or the temperature is decreased a visible departure from ideal behaviour is seen also a difference between ideal and non-ideal gas is seen. We can see the example of the type of deviation that occurs in Boyle’s law for H2 at room temperature.
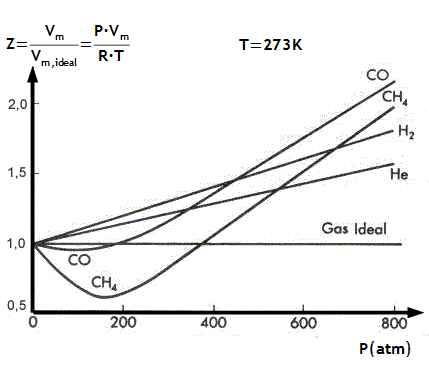
The deviations or the difference between ideal gas and real gas can be displayed more distinctively by plotting the graph with the ratio of the observed molar volume Vm to the ideal molar volume Vm ideal( = RT/p) as the function of pressure at constant temperature and this ratio is called the compression factor Z and this can be expressed as
Z = Vm/ Vm ideal = pVm/RT
The major difference between ideal gas and real gas is that for an ideal gas, Z = 1 and is independent of pressure and temperature. For example a real gas, Z = f(T,p) a function of both temperature and pressure as shown in figure below a graph between Z and p for some gases at 273.15 K, the inference we can conclude is that
Related Topics, |
- Difference between ideal gas and real gas is that in real gas Z the compression factor is always greater than 1 for H2 because the attractive interactions are so weak that the repulsive interactions dominate even at low pressures.
- For CH4, at low pressure, Z is less than 1. That is, their molar volumes of the methane gas are smaller than that of a perfect gas, showing that the molecules are drawn together slightly and also, we can conclude that for these molecules and these conditions the attractive interactions are more dominant and this is the differentiate between real gas and ideal gas.
In the below figure, it gives the impression that the nature of deviations depends upon the nature of the gas. The more important determining factor is the temperature relative to the critical temperature of a selected/ particular gas which is near the critical temperature in real gas.
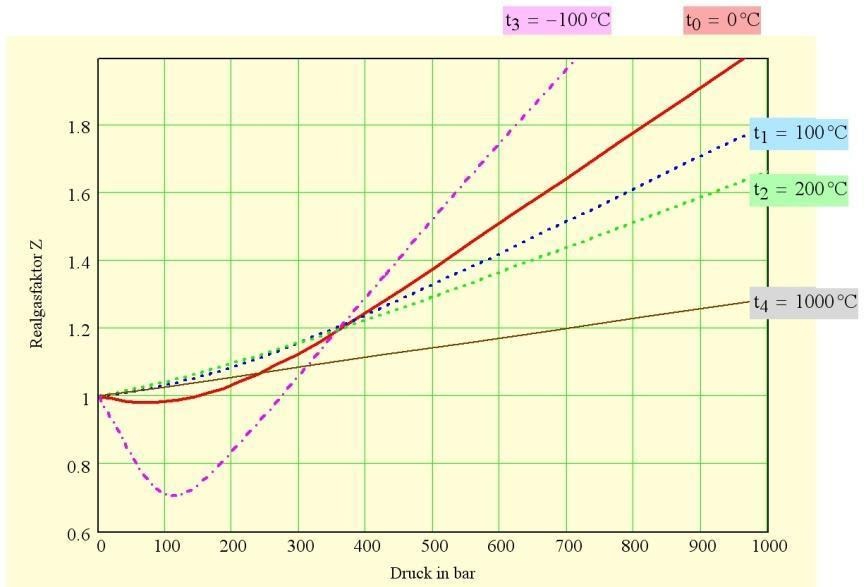
Given the pressure is of the order of 1 bar or less, and the temperature is not too near the point of liquefaction, the observed deviations from the ideal gas laws are not more than a few % . Thus under these conditions, the equation pV =nRT and related expressions may be used. These are some of the examples of ideal gas and real gas.
The ideal gas differentiate from real gas or the difference between ideal gas and real gasis because ideal gas deviates from its ideal behaviour and this is because the ideal gas can be derived from the kinetic theory of gases which is based on the following two assumptions:
- The volume occupied by the molecules is negligible in comparison to the total volume of the gas in consideration.
- The molecules exert no forces of attraction on one another molecule.
As we know neither of these assumptions can be regarded as applicable to real gases that the latter show departure from the ideal gas behaviour.
The person who was the first to systematically introduce the correction terms due to the above two invalid assumptions in the ideal gas equation piVi = nRT was Van der Waals.
His corrections are
- Correction in Volume Vi = V – nb where b is excluded or co- volume.
- Correction for forces of attraction.
The equation which is applicable to all real gas is and is known as van der waals equation.
(p + n2a / V2) ( V- nb) = nRT
Thus, due to this, there is major difference between real and ideal gas.
Also, students can refer,
- NCERT solutions for Class 11 Chemistry Chapter 5 States of Matter
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 5 States of Matter
- NCERT notes Class 11 Chemistry Chapter 5 States of Matter
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Non ideal gases are gases which do not obey the ideal gas laws exactly under all conditions of temperature and pressure.
A hypothetical gas that obeys the gas law exactly under all conditions of temperature and pressure is perfect gas or ideal gas.
The real molecules possess finite size and do not overlap and thus has a strong, short – ranged repulsion between them.
Ideal gas and real gas have major difference of: A hypothetical gas that obeys the gas law exactly under all conditions of temperature and pressure is ideal gas and real gas is a gas that does not follow the assumptions made according to kinetic – molecular theory.
The ratio of the observed molar volume Vm to the ideal molar volume Vm ideal( = RT/p) as the function of pressure at constant temperature and this ratio is called the compression factor Z.
Also Read
02 Jul'25 08:05 PM
02 Jul'25 07:59 PM
02 Jul'25 07:55 PM
02 Jul'25 06:31 PM
02 Jul'25 06:31 PM
02 Jul'25 06:31 PM
02 Jul'25 06:28 PM
02 Jul'25 06:28 PM
02 Jul'25 06:28 PM
02 Jul'25 06:28 PM

