Coefficient of Viscosity - Definition, Formula, Types, Application, FAQs
Viscosity is defined as the degree to which a fluid resists flow when subjected to a force; it is calculated by dividing the tangential friction force acting per unit area by the velocity gradient under streamlined flow circumstances. Viscosity is an important rheological measurement that is directly related to flow resistance. A fluid's viscosity is defined as its resistance to flow.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
This Story also Contains
- Viscosity Measurement
- The Coefficient of Viscosity Formula
- Numerically
- Applications of Viscosity
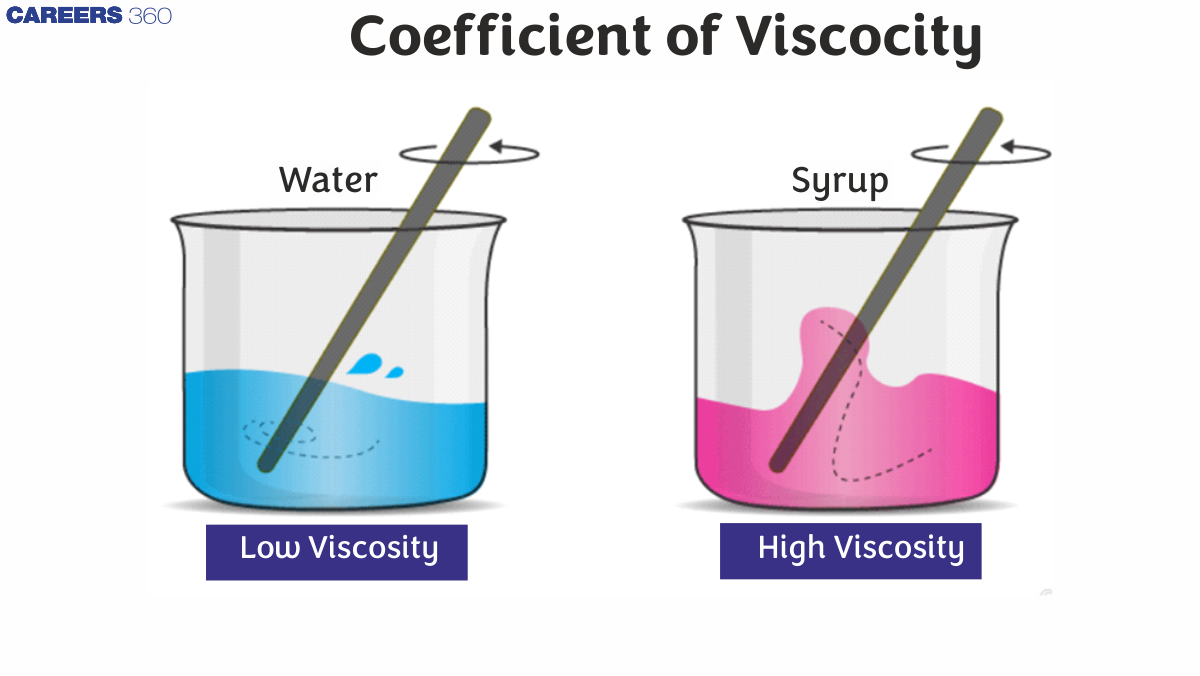
The coefficient of viscosity of liquids such as the coefficient of viscosity of water, alcohols, petrol, and others flow more readily and quickly than glycerin solution, honey, and oil. This is due to its viscosity, which is a physical attribute. It illustrates the fluid's flow resistance in simple terms.
Viscosity Measurement
The coefficient of viscosity is used to calculate the viscosity. It is constant for a liquid and is determined by the type of the liquid. When a liquid flows through a tube at varied pressures, Poiseuille's method is formally employed to estimate the coefficient of viscosity.
Fluid's coefficient of viscosity decreases as temperature rises, whereas gases' coefficient of viscosity rises in the opposite direction. While the coefficient of viscosity of gases increases as the temperature rises. The fluid's temperature rises, loosening the connections between molecules. These bonds are directly related to viscosity, resulting in a drop in the coefficient.
Commonly Asked Questions
The Coefficient of Viscosity Formula
The coefficient of viscosity is the ratio of the shearing force to the fluid's velocity gradient.
The symbol of the coefficient of viscosity is η. As a result, the viscosity coefficient is given by,
η = F.d / A .ⅴ
Here, F is the tangential force needed to maintain a unit velocity gradient between two parallel liquid layers of equal area.
v stands for velocity.
A stands for the area.
d is the distance between the two liquid layers skidding over one another.
The velocity gradient is defined as the differential in velocity between neighboring layers of a fluid stream.
Also read :
Commonly Asked Questions
Numerically
Coefficient of viscosity (η) = Fd/Av, where F denotes extraneous power, d denotes layer separation, and v denotes speed.
Force Dimensional Formula = M¹L¹T-²
Area Dimensional Formula = MºL²Tº
Distance Formula in Dimensions = MºL¹Tº
MºL¹T-¹is the dimensional formula for velocity.
Combining these traits in the preceding criteria we get
[η]= [M¹L¹T-²] [MºL¹Tº]/[MºL²Tº] [MºL¹T-¹] = [M¹L-¹T-¹]
Types of Viscosity
Dynamic and kinematic viscosities are the two distinct estimates of viscosity used to depict fluids.
These depict the growth of the liquid in various ways viscosity depends on how they are approximated; nevertheless, if the liquid viscosity is known, they are compatible.
Unique viscosity and kinematic viscosity v are the two types of viscosity that are commonly used.
Dynamic Viscosity
The shear worry to the shear rate for a liquid is measured using dynamic viscosity.
The condition μ = ρν where ρ is the viscosity of the liquid, identifies dynamic viscosity with kinematic viscosity.
The centipoise is the unit of dynamic viscosity is μ. If liquid viscosity is measured in grams per cubic centimeter, kinematic viscosity is measured in centistoke.
As a result, 1 centistoke becomes 1 centipoise when separated by 1 g/cc.
Kinematic Viscosity
The ratio of viscous power to inertial power on a liquid is measured by kinematic viscosity.
The diffusivity of mass as well as warmth which is the diffusivity of energy, can be compared to kinematic viscosity.
Applications of Viscosity
- Vehicles with oil
When it comes to putting oil in the car or truck, one should be aware of its viscosity. This is because factors affecting viscosity grating, and erosion factors affecting viscosity heat as a result.
Some oils have a constant viscosity, while others respond to heat or cold; if the oil's viscosity list is low, it may become thinner as it warms, causing problems when the car is working on a hot summer day.
Cooking
Viscosity plays an important role in the preparation and serving of food. Cooking oils viscosity can alter as they heat, and many become significantly more viscous as they cool.
When fats are warmed, they become viscous, but when they are cold, they become powerful. The viscosity of sauces, soups, and stews also influences different cooking styles.
Assembling
To run smoothly, adequate oil is required while assembling hardware. Oils that are excessively thick can clog pipelines and cause them to shut down. Ointments that are excessively thin provide insufficient protection for moving parts.
Gel Medicine (Medication)
As liquids are injected into the body intravenously, viscosity is crucial in medicine.
Blood viscosity is a significant problem: excessively thick blood might form dangerous internal clusters, but blood that is too thin won't clump, resulting in dangerous blood misfortune and even death.
Also read -
| NCERT Solutions for Class 11 Chemistry | NCERT notes Class 11 Chemistry |
| NCERT Solutions for Class 12 Chemistry | NCERT notes Class 12 Chemistry |
| NCERT Solutions for All Subjects | NCERT Notes For All Subjects |
Frequently Asked Questions (FAQs)


