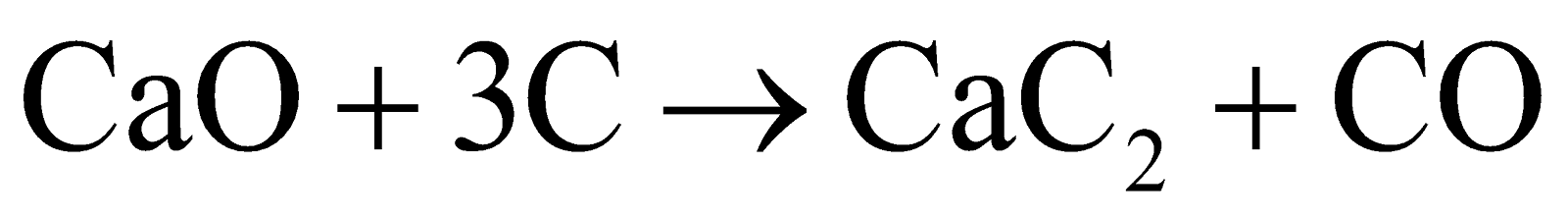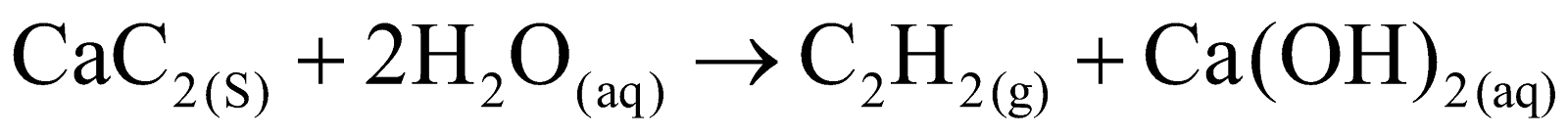1. What is meant by carbide?
It is a chemical compound which consists of carbon and metallic or semi-metallic elements. It is the ionic or covalent bond that connects the carbide group to metallic or semi-metallic elements.
2. Give some properties of carbides
Carbides generally possess very high melting point, they conduct heat and electricity. Carbides are compounds with metallic lustre property.
3. Why is calcium carbide considered an important industrial chemical?
Calcium carbide is an important industrial chemical primarily due to its use in producing acetylene gas. Acetylene is widely used in welding and cutting metals, and as a precursor in various organic syntheses. Additionally, calcium carbide is used in the production of calcium cyanamide, a fertilizer.
4. What role does calcium carbide play in the nitrogen cycle when used in agriculture?
Calcium carbide doesn't directly participate in the nitrogen cycle. However, it's used to produce calcium cyanamide (CaCN2), a fertilizer that can be converted to ammonia in soil. This ammonia then enters the nitrogen cycle, providing a source of nitrogen for plants.
5. What safety precautions are necessary when handling calcium carbide?
When handling calcium carbide, it's crucial to avoid contact with water or moisture. Protective gear like gloves and goggles should be worn. It should be stored in a dry, well-ventilated area away from water sources. Proper ventilation is necessary when using it, as the acetylene produced is flammable.
6. How does the reaction of calcium carbide with water demonstrate Le Chatelier's principle?
The reaction of calcium carbide with water is essentially irreversible under normal conditions, so Le Chatelier's principle doesn't significantly apply here. However, if we consider the dissolution of the produced calcium hydroxide, adding more water would shift that equilibrium towards more dissolution, demonstrating Le Chatelier's principle.
7. Why is the production of calcium carbide considered an energy-intensive process?
Producing calcium carbide is energy-intensive because it requires very high temperatures (around 2000-2100°C) to react calcium oxide with carbon. This high energy requirement is needed to break existing bonds and form the new calcium-carbon bonds in calcium carbide.
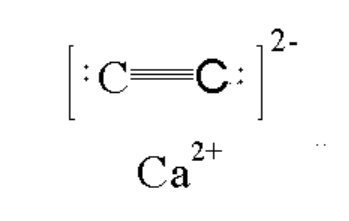
8. What is the significance of the C-C triple bond in the carbide ion of calcium carbide?
The C-C triple bond in the carbide ion (C22-) is crucial to calcium carbide's reactivity and its ability to produce acetylene. This strong, short bond stores significant energy, which is released when the compound reacts with water, facilitating the formation of the triple bond in acetylene.
9. How does the electronegativity difference between calcium and carbon affect the nature of calcium carbide?
The large electronegativity difference between calcium (low electronegativity) and carbon (high electronegativity) results in a highly ionic bond between Ca2+ and C22- in calcium carbide. This ionic character contributes to its high melting point and its reactivity with polar molecules like water.
10. How does the thermal stability of calcium carbide compare to other metal carbides?
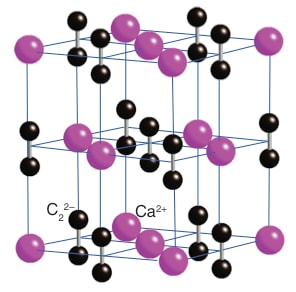
Calcium carbide is relatively thermally stable compared to many other metal carbides. It has a high melting point (2160°C) and doesn't decompose at normal temperatures. This stability is due to the strong ionic bonding between Ca2+ and C22- ions and the triple bond in the C22- ion.
11. How does the reactivity of calcium carbide demonstrate the trends in reactivity of Group 2 elements?
Calcium carbide's high reactivity with water aligns with the general trend of increasing reactivity down Group 2. As a compound of calcium, which is below beryllium and magnesium in the group, calcium carbide shows more vigorous reactions with water compared to beryllium or magnesium compounds.
12. How does the solubility of calcium carbide compare to other calcium compounds?
Calcium carbide is not soluble in water; instead, it reacts with water. This behavior differs from many other calcium compounds like calcium chloride or calcium nitrate, which are highly soluble in water. The reactivity of the C22- ion with water prevents true dissolution.
13. How does the structure of calcium carbide relate to its reactivity?
Calcium carbide has an ionic structure with Ca2+ cations and C22- anions. The C22- anion contains a triple bond between the carbon atoms, making it highly reactive. This structure allows calcium carbide to readily react with water, producing acetylene gas and calcium hydroxide.
14. How does the reactivity of calcium carbide compare to other calcium compounds?
Calcium carbide is generally more reactive than many other calcium compounds, particularly with water. This heightened reactivity is due to the presence of the highly reactive C22- anion, which is not found in common calcium compounds like calcium carbonate or calcium chloride.
15. What role does calcium's electron configuration play in the formation of calcium carbide?
Calcium's electron configuration ([Ar] 4s2) allows it to easily lose two electrons, forming a Ca2+ ion. This ability to form a stable 2+ ion enables calcium to bond with the C22- anion in calcium carbide, demonstrating the characteristic behavior of s-block elements in forming ionic compounds.
16. How does the bonding in calcium carbide differ from that in calcium metal?
In calcium metal, atoms are held together by metallic bonding, with delocalized electrons moving freely. In calcium carbide, there's ionic bonding between Ca2+ and C22- ions, as well as a covalent triple bond between the carbon atoms in the C22- ion, resulting in a very different structure and properties.
17. Why does calcium carbide produce acetylene when it reacts with water?
The reaction of calcium carbide with water is a hydrolysis reaction. The C22- anion in calcium carbide accepts protons from water, forming acetylene (C2H2) gas. Meanwhile, the Ca2+ ions form calcium hydroxide with the remaining hydroxide ions from water.
18. What is calcium carbide and why is it important in chemistry?
Calcium carbide (CaC2) is an inorganic compound composed of calcium and carbon. It's important in chemistry due to its reactivity with water, producing acetylene gas, which has various industrial applications. This compound demonstrates the unique properties of s-block elements and their ability to form ionic compounds with non-metals.
19. How does the production of calcium carbide relate to the reactivity of calcium?
Calcium carbide is produced by heating calcium oxide (lime) with carbon at high temperatures. This process showcases calcium's high reactivity, as it readily combines with carbon under these conditions to form the carbide compound, demonstrating the strong reducing properties of calcium.
20. Why is calcium carbide stored in airtight containers?
Calcium carbide is stored in airtight containers to prevent contact with moisture in the air. If exposed to water vapor, it slowly reacts to produce acetylene gas and calcium hydroxide, which can be dangerous if allowed to accumulate and can degrade the quality of the calcium carbide.
21. How does the reaction of calcium carbide with water demonstrate the concept of a double displacement reaction?
The reaction of calcium carbide with water is not actually a double displacement reaction. It's a hydrolysis reaction where water molecules break down the calcium carbide. A true double displacement reaction involves the exchange of ions between two compounds, which doesn't occur in this case.
22. Why doesn't calcium form a simple carbide like many transition metals do?
Unlike transition metals that can form simple interstitial carbides, calcium forms an ionic carbide due to its larger atomic size and lower number of valence electrons. Calcium's tendency to completely lose its valence electrons leads to the formation of the distinct C22- ion rather than carbon atoms fitting into metal lattice interstices.
23. How does the crystal structure of calcium carbide influence its properties?
Calcium carbide has a rock-salt crystal structure, with Ca2+ and C22- ions arranged in a cubic lattice. This structure contributes to its high melting point and brittleness. The arrangement of ions also influences its reactivity, as it allows water molecules to easily access and react with the C22- ions.
24. What is the environmental impact of calcium carbide production and use?
Calcium carbide production can have significant environmental impacts. The high-temperature process requires substantial energy, often from fossil fuels, contributing to carbon emissions. Its reaction with water produces acetylene, a greenhouse gas. Proper handling and disposal are crucial to prevent water contamination.
25. Why is calcium carbide sometimes used as a ripening agent for fruits?
Calcium carbide is sometimes (illegally) used as a fruit ripening agent because it produces acetylene gas when it reacts with moisture. Acetylene is similar in structure to ethylene, a natural plant hormone that induces ripening. However, this practice is dangerous due to toxic impurities in industrial calcium carbide.
26. How does the reaction of calcium carbide with nitrogen demonstrate the reactivity of s-block elements?
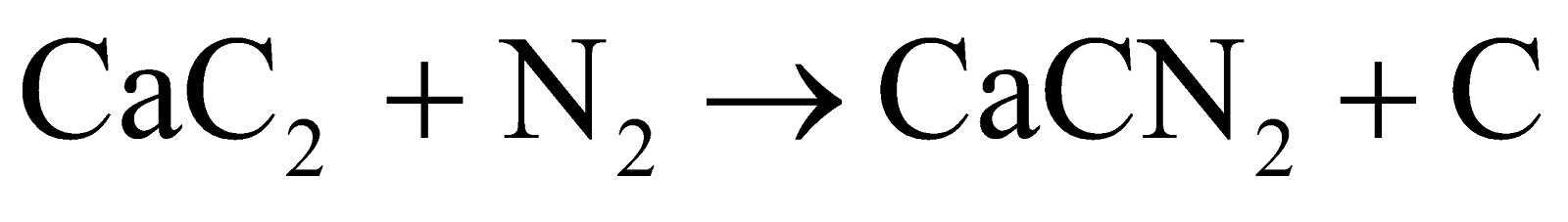
Calcium carbide reacts with nitrogen at high temperatures to form calcium cyanamide (CaCN2). This reaction showcases the ability of s-block elements like calcium to form compounds with various non-metals under appropriate conditions, demonstrating their high reactivity and versatile chemistry.
27. What is the relationship between the structure of calcium carbide and its brittleness?
The brittleness of calcium carbide is related to its ionic crystal structure. The strong electrostatic forces between Ca2+ and C22- ions create a rigid lattice. When stress is applied, the like-charged ions align, causing repulsion and leading to brittle fracture rather than plastic deformation.
28. How does the reactivity of calcium carbide with acids compare to its reactivity with water?
Calcium carbide reacts more vigorously with acids than with water. In both cases, acetylene is produced, but the reaction with acids is faster due to the higher concentration of H+ ions. With strong acids, the reaction can be explosive, highlighting the importance of proper handling.
29. Why is the production of calcium carbide considered a redox reaction?
The production of calcium carbide (CaO + 3C → CaC2 + CO) is a redox reaction because there's a transfer of electrons. Carbon is reduced (gains electrons) to form C22-, while some carbon is oxidized to CO. The calcium maintains its oxidation state of +2 throughout the reaction.
30. How does the presence of impurities in calcium carbide affect its reactivity?
Impurities in calcium carbide can significantly affect its reactivity. Common impurities like calcium phosphide can produce toxic phosphine gas when the carbide reacts with water. Other impurities may catalyze side reactions or alter the rate of acetylene production, affecting the compound's performance in various applications.
31. What role does calcium carbide play in the production of calcium cyanamide fertilizer?
Calcium carbide is a key intermediate in producing calcium cyanamide fertilizer. It reacts with pure nitrogen gas at high temperatures to form calcium cyanamide: CaC2 + N2 → CaCN2 + C. This reaction showcases how calcium carbide can be used to fix atmospheric nitrogen into a form usable by plants.
32. How does the electron affinity of carbon influence the formation of the C22- ion in calcium carbide?
Carbon's relatively high electron affinity allows it to accept electrons from calcium to form the C22- ion. However, the formation of C22- is not solely due to electron affinity; it's also influenced by the strong Ca-C ionic bond and the stability gained from the C-C triple bond in the C22- ion.
33. Why doesn't calcium form a carbonate instead of a carbide when reacted with carbon?
Calcium doesn't form a carbonate when reacted with carbon because the reaction conditions (high temperature, absence of oxygen) favor carbide formation. Carbonates require oxygen, which is deliberately excluded in carbide production. The high temperature also decomposes any carbonate that might form.
34. How does the atomic radius of calcium influence its ability to form calcium carbide?
Calcium's relatively large atomic radius allows it to accommodate the large C22- ion in its crystal structure. The size of calcium atoms enables efficient packing with the carbide ions, contributing to the stability of the compound. This is in contrast to smaller elements that might not form stable carbides with this structure.
35. What is the significance of calcium carbide in understanding the chemistry of acetylides?
Calcium carbide is a classic example of an acetylide compound, where the C22- ion is present. It serves as a model for understanding the properties and reactions of acetylides in general, including their ability to generate acetylene upon hydrolysis and their use in organic synthesis.
36. How does the formation of calcium carbide demonstrate the concept of lattice energy?
The formation of calcium carbide involves strong ionic bonding between Ca2+ and C22- ions, releasing significant lattice energy. This high lattice energy contributes to the compound's stability and high melting point. The magnitude of this energy is influenced by the charges and sizes of the ions involved.
37. Why is calcium carbide sometimes called "carbide lime," and how does this relate to its production?
Calcium carbide is sometimes called "carbide lime" because it's produced from lime (calcium oxide) and carbon. This name reflects its manufacturing process and helps distinguish it from other carbides. It also hints at the alkaline nature of its hydrolysis products, similar to the alkalinity of lime.
38. How does the reaction of calcium carbide with water demonstrate the concept of a limiting reagent?
In the reaction of calcium carbide with water, either compound can be the limiting reagent depending on their relative quantities. If water is in excess, all calcium carbide will react, producing the maximum possible amount of acetylene. If calcium carbide is in excess, the reaction will stop when all water is consumed.
39. What is the relationship between the structure of calcium carbide and its ability to form acetylene?
The structure of calcium carbide, with its C22- ion containing a triple bond, is key to its ability to form acetylene. When water protonates the C22- ion, the triple bond is preserved, forming HC≡CH (acetylene). This direct transfer of the triple bond structure is what makes calcium carbide an efficient acetylene precursor.
40. How does the concept of oxidation states apply to the carbon atoms in calcium carbide?
In calcium carbide (CaC2), the oxidation state of carbon is -1. This is because the two carbon atoms share a total charge of -2 (to balance the Ca2+ ion), and this charge is equally distributed between them. This oxidation state is unusual for carbon and contributes to the compound's unique reactivity.
41. Why doesn't calcium carbide conduct electricity in its solid state, despite being an ionic compound?
Although calcium carbide is an ionic compound, it doesn't conduct electricity in its solid state because the ions are fixed in the crystal lattice and cannot move freely. Electrical conductivity in ionic compounds typically requires the ions to be mobile, which occurs in aqueous solutions or when the compound is molten.
42. How does the reaction of calcium carbide with water demonstrate the concept of enthalpy change?
The reaction of calcium carbide with water is exothermic, releasing heat. This demonstrates a negative enthalpy change, where the products (acetylene and calcium hydroxide) have less chemical energy than the reactants. The heat released is due to the formation of new, stable bonds in the products.
43. What role does calcium carbide play in understanding the periodic trends of Group 2 elements?
Calcium carbide helps illustrate periodic trends in Group 2, particularly increasing reactivity down the group. The ability of calcium to form this reactive carbide, which vigorously produces acetylene with water, contrasts with the less reactive behavior of elements above it (beryllium and magnesium) in similar scenarios.
44. How does the production of calcium carbide relate to the concept of Gibbs free energy?
The production of calcium carbide (CaO + 3C → CaC2 + CO) is non-spontaneous at room temperature, requiring high temperatures to proceed. This relates to Gibbs free energy as the reaction becomes spontaneous (ΔG < 0) only at elevated temperatures where the increase in entropy (TΔS term) overcomes the positive enthalpy change.
45. Why is calcium carbide considered a reducing agent, and how does this property relate to its electronic structure?
Calcium carbide acts as a reducing agent due to the presence of the C22- ion, which can donate electrons in reactions. This reducing property stems from the relatively high-energy electrons in the carbon-carbon triple bond, which can be transferred to other species in redox reactions.
46. How does the reaction of calcium carbide with heavy water (D2O) differ from its reaction with regular water?
The reaction of calcium carbide with heavy water (D2O) produces deuterated acetylene (D-C≡C-D) instead of regular acetylene. The reaction mechanism is the same, but the product contains deuterium instead of hydrogen. This reaction is slower due to the kinetic isotope effect caused by the heavier deuterium atoms.
47. What is the significance of calcium carbide in understanding the concept of ionic radii in chemical compounds?
Calcium carbide provides an interesting case study for ionic radii. The large size of the C22- ion compared to common anions like Cl- or O2- influences the crystal structure and properties of the compound. It demonstrates how ionic size affects packing in crystal lattices and overall compound stability.
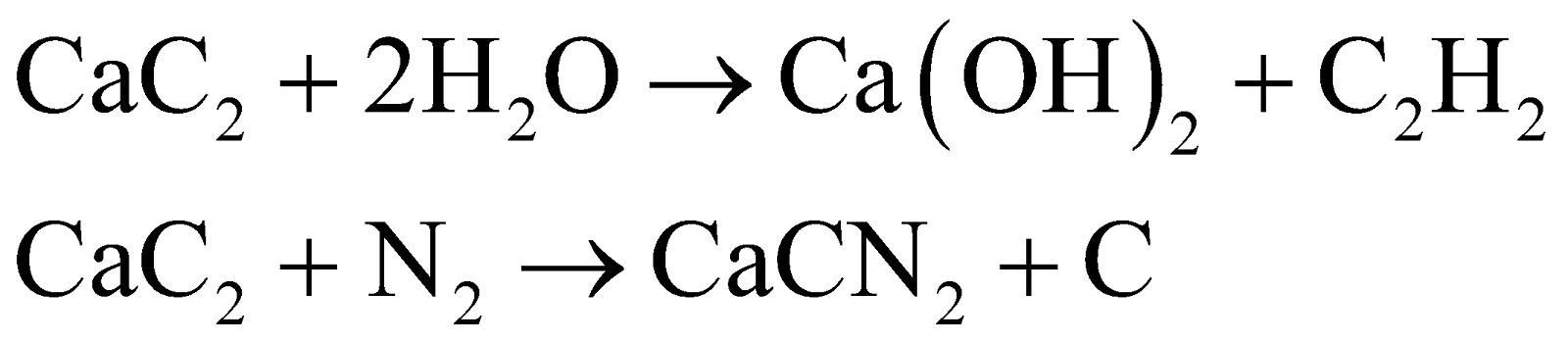
![]()
![]()
![]()
![]()
![]() of CaC2. The rest of it is composed of CaO, Ca3P2, CaS, Ca3N2, SiC etc.
of CaC2. The rest of it is composed of CaO, Ca3P2, CaS, Ca3N2, SiC etc.![]() . This is an endothermic reaction that requires 110 kilocalories per mole and very high temperature to expel CO.
. This is an endothermic reaction that requires 110 kilocalories per mole and very high temperature to expel CO.