1. What is kp and kc?/what is kp?/ Write kp meaning.
Kp constant of equilibrium w.r.t. the atmospheric pressure and Kc is the constant of equilibrium w.r.t. the molar concentration of the gas mixture.
2. What is the relation between kp kc and kx on the basis of pressure?
Kx depends on the atmospheric pressure while Kp and Kc are independent of the pressure.
3. What does the ka and kb relationship determine?
Ka and Kb determine the dissociation property of acid-base.
4. Write the difference between kp and kc.
Kp is the constant of equilibrium applicable in the partial pressures while Kc is the constant of equilibrium applicable in the concentrations.
5. What is the relationship between Kp and Kc?
Kp and Kc are equilibrium constants related to gas-phase reactions. Kp is the equilibrium constant in terms of partial pressures, while Kc is in terms of concentrations. They are related by the equation Kp = Kc(RT)^Δn, where R is the gas constant, T is temperature, and Δn is the change in moles of gas.
6. Why do we need both Kp and Kc?
We use both Kp and Kc because some reactions are more conveniently expressed in terms of pressure (Kp), while others are better described using concentration (Kc). The choice depends on the specific reaction and experimental conditions.
7. How does the value of Δn affect the relationship between Kp and Kc?
Δn is the change in the number of moles of gas during the reaction. If Δn = 0, then Kp = Kc. If Δn > 0, Kp > Kc, and if Δn < 0, Kp < Kc. This is because gases expand or contract based on the number of moles present.
8. Can Kp and Kc ever be equal?
Yes, Kp and Kc are equal when Δn = 0, meaning there is no change in the number of moles of gas during the reaction. This occurs in reactions where the number of gaseous reactant molecules equals the number of gaseous product molecules.
9. How does temperature affect the relationship between Kp and Kc?
Temperature appears in the relationship Kp = Kc(RT)^Δn. As temperature increases, the difference between Kp and Kc becomes more pronounced for reactions where Δn ≠ 0. For isothermal processes, the relationship remains constant.
10. What units are used for Kp and Kc?
Kp is typically expressed in units of pressure (e.g., atm, Pa), while Kc is expressed in units of concentration (e.g., mol/L). However, in many calculations, these constants are treated as dimensionless.
11. How do you convert between Kp and Kc?
To convert between Kp and Kc, use the equation Kp = Kc(RT)^Δn. You need to know the temperature, the gas constant R, and Δn (change in moles of gas) for the reaction. Rearrange the equation to solve for the unknown constant.
12. What is the significance of Δn in gas-phase reactions?
Δn represents the change in the number of moles of gas during a reaction. It's crucial for understanding how the reaction affects gas volume and pressure, and it determines the relationship between Kp and Kc.
13. How does the ideal gas law relate to Kp and Kc?
The ideal gas law (PV = nRT) is fundamental to the relationship between Kp and Kc. It allows us to convert between pressure and concentration, which is why we can relate Kp and Kc using the equation Kp = Kc(RT)^Δn.
14. Why is R (gas constant) included in the Kp-Kc relationship?
The gas constant R is included because it's a fundamental constant in the ideal gas law, which relates pressure, volume, and temperature for gases. It allows us to convert between pressure and concentration units consistently.
15. How does the volume of the reaction vessel affect Kp and Kc?
The volume of the reaction vessel directly affects Kc (concentration-based) but not Kp (pressure-based). Changing the volume will change concentrations but not partial pressures, so Kc may change while Kp remains constant.
16. Can you use Kp for reactions involving solids or liquids?
Kp is specifically for gas-phase reactions. For reactions involving solids or liquids, we typically use Kc. Solids and liquids don't have "partial pressures" in the same way gases do, so Kp isn't applicable for them.
17. How do you determine Δn for a reaction?
To determine Δn, subtract the number of moles of gaseous reactants from the number of moles of gaseous products in the balanced chemical equation. Δn = (moles of gaseous products) - (moles of gaseous reactants).
18. What happens to the Kp-Kc relationship in non-ideal gas conditions?
The relationship Kp = Kc(RT)^Δn assumes ideal gas behavior. In non-ideal conditions (high pressure, low temperature), this relationship becomes less accurate, and corrections may be needed using more complex equations of state.
19. How does the magnitude of Kp compare to Kc for a reaction where Δn is positive?
When Δn is positive (more moles of gas products than reactants), Kp will be greater than Kc. This is because Kp = Kc(RT)^Δn, and (RT)^Δn will be greater than 1 for positive Δn.
20. Can Kp and Kc have different signs?
No, Kp and Kc cannot have different signs. Both are always positive for any real equilibrium. If a calculation results in a negative value, it indicates an error or that the reaction proceeds in the opposite direction.
21. How do catalysts affect the relationship between Kp and Kc?
Catalysts do not affect the equilibrium constants Kp or Kc, nor their relationship. Catalysts only speed up the rate at which equilibrium is reached but do not change the equilibrium position or constants.
22. What's the importance of standard conditions in Kp and Kc calculations?
Standard conditions (usually 1 atm pressure and 25°C) provide a consistent reference point for comparing equilibrium constants. When converting between Kp and Kc, it's important to use the actual temperature of the reaction, not necessarily standard conditions.
23. How do you handle Kp and Kc for reactions with multiple gases?
For reactions with multiple gases, Kp and Kc are still related by Kp = Kc(RT)^Δn. Δn is calculated considering all gaseous species, and each gas contributes to the overall equilibrium constant based on its stoichiometric coefficient.
24. Can you use Kp and Kc interchangeably in equilibrium calculations?
While Kp and Kc are related, they cannot be used interchangeably. You must use the appropriate constant based on whether you're working with pressures (Kp) or concentrations (Kc), and convert between them if necessary.
25. How does the presence of an inert gas affect Kp and Kc?
An inert gas doesn't affect Kc because it doesn't change concentrations. However, it can affect Kp by changing total pressure. The partial pressures of reacting gases may change, potentially affecting Kp, even though the equilibrium itself isn't shifted.
26. What role does Le Chatelier's principle play in understanding Kp and Kc?
Le Chatelier's principle helps predict how changes in conditions affect equilibrium. While it doesn't directly involve Kp or Kc, understanding this principle is crucial for predicting how changes in pressure or concentration might affect the equilibrium described by these constants.
27. How do you interpret very large or very small values of Kp or Kc?
A very large K value (Kp or Kc >> 1) indicates the equilibrium strongly favors products. A very small K value (K << 1) indicates the equilibrium strongly favors reactants. The magnitude of K gives insight into the extent of the reaction at equilibrium.
28. Can Kp and Kc be used for non-equilibrium situations?
No, Kp and Kc are specifically equilibrium constants. They only apply when a system has reached chemical equilibrium. For non-equilibrium situations, we use reaction quotients (Qp and Qc) which have the same form but represent non-equilibrium conditions.
29. How does pressure affect Kp and Kc differently?
Changes in total pressure directly affect Kp but not Kc. Increasing pressure can change the partial pressures of gases, potentially changing Kp. However, Kc remains constant with pressure changes as it depends on concentrations, not pressures.
30. What's the significance of the (RT)^Δn term in the Kp-Kc relationship?
The (RT)^Δn term accounts for the conversion between pressure and concentration units. It also reflects how the relationship between Kp and Kc depends on both temperature and the change in the number of moles of gas during the reaction.
31. How do you handle Kp and Kc for reactions at very high temperatures?
At very high temperatures, gases may deviate significantly from ideal behavior. In such cases, the simple Kp = Kc(RT)^Δn relationship may not hold accurately. More complex equations of state or empirical corrections may be necessary.
32. Can Kp and Kc change with time for a given reaction?
No, for a given reaction at a specific temperature, Kp and Kc are constants. They do not change with time once equilibrium is established. However, they can change if the temperature changes or if the reaction conditions are altered.
33. How do you determine whether to use Kp or Kc for a specific problem?
Choose Kp when the problem provides or asks for information in terms of pressures. Use Kc when dealing with concentrations. If you have one but need the other, you can convert between them using the Kp = Kc(RT)^Δn relationship.
34. What's the relationship between Kp, Kc, and the Gibbs free energy change (ΔG)?
Both Kp and Kc are related to the standard Gibbs free energy change (ΔG°) by the equation ΔG° = -RT ln(K). This relationship shows how equilibrium constants are fundamentally connected to the thermodynamics of the reaction.
35. How do you handle Kp and Kc for reactions involving very low gas concentrations?
For very low gas concentrations, ideal gas behavior is usually a good approximation, so the standard Kp = Kc(RT)^Δn relationship typically holds. However, experimental measurements may become challenging, requiring sensitive equipment.
36. Can Kp and Kc be used to predict the direction of a reaction?
Yes, by comparing the reaction quotient (Q) to K (either Kp or Kc). If Q < K, the reaction will proceed forward. If Q > K, it will proceed backward. If Q = K, the system is at equilibrium.
37. How do Kp and Kc relate to the concept of chemical potential?
Chemical potential (μ) is related to the partial pressure or concentration of a species. The equilibrium constants Kp and Kc represent the balance of chemical potentials at equilibrium, where the total Gibbs free energy is minimized.
38. What's the significance of the standard state in Kp and Kc calculations?
The standard state (usually 1 atm for gases, 1 M for solutions) provides a reference point for calculations. Kp and Kc values are often reported for standard conditions, allowing for consistent comparisons between different reactions.
39. How do you handle Kp and Kc for coupled reactions?
For coupled reactions, the overall Kp or Kc is the product of the individual equilibrium constants. This is because the logarithm of K is additive for coupled reactions, reflecting the additive nature of Gibbs free energy changes.
40. Can you use Kp and Kc to calculate equilibrium concentrations or pressures?
Yes, given initial conditions and the value of Kp or Kc, you can set up equations to solve for equilibrium concentrations or pressures. This often involves using an ICE table (Initial, Change, Equilibrium) and solving algebraic equations.
41. How do Kp and Kc relate to the concept of fugacity in non-ideal gases?
For non-ideal gases, fugacity (f) replaces pressure (P) in thermodynamic equations. In these cases, we might use Kf (equilibrium constant in terms of fugacity) instead of Kp. Kf relates to Kc similarly to how Kp does, but accounts for non-ideal behavior.
42. What's the importance of dimensional analysis in Kp and Kc calculations?
Dimensional analysis is crucial in Kp and Kc calculations to ensure consistency in units. While these constants are often treated as dimensionless, keeping track of units helps prevent errors, especially when converting between Kp and Kc.
43. How do you handle Kp and Kc for reactions involving gases dissolved in liquids?
For gases dissolved in liquids, Henry's law often applies, relating the concentration of the dissolved gas to its partial pressure above the solution. In such cases, a combination of Kp (for the gas phase) and Kc (for the solution) might be necessary.
44. Can Kp and Kc be used to calculate the yield of a reaction?
Yes, Kp and Kc can be used to calculate the theoretical yield of a reaction at equilibrium. By knowing the initial concentrations or pressures and the equilibrium constant, you can determine the equilibrium composition and thus the yield.
45. How do Kp and Kc relate to the concept of activity in non-ideal solutions?
In non-ideal solutions, activity (a) replaces concentration in thermodynamic equations. The equilibrium constant in terms of activities (Ka) is used instead of Kc. Ka is more fundamental and applies to both ideal and non-ideal systems.
46. What's the significance of the temperature dependence of Kp and Kc?
The temperature dependence of Kp and Kc is described by the van 't Hoff equation, which relates the change in K to the enthalpy change of the reaction. This allows prediction of how equilibrium constants change with temperature.
47. How do you handle Kp and Kc for reactions involving multiple phases?
For reactions involving multiple phases, you typically use Kc and include only the species in the phase where the reaction occurs. For gas-phase components, you can convert partial pressures to concentrations if needed.
48. Can Kp and Kc be used to calculate the pH of a solution?
Yes, for reactions involving acids and bases, Kc (often denoted as Ka or Kb for acids and bases) can be used to calculate pH. The equilibrium constant is related to the dissociation of the acid or base, which determines the H+ concentration and thus pH.
49. How do Kp and Kc relate to the concept of reaction quotient Q?
The reaction quotient Q has the same form as Kp or Kc but uses non-equilibrium concentrations or pressures. At equilibrium, Q equals K. Comparing Q to K allows prediction of the direction in which a reaction will proceed to reach equilibrium.
50. What's the significance of the standard enthalpy and entropy changes in relation to Kp and Kc?
The standard enthalpy (ΔH°) and entropy (ΔS°) changes are related to Kp and Kc through the Gibbs free energy change: ΔG° = ΔH° - TΔS° = -RT ln(K). This relationship allows prediction of equilibrium constants from thermodynamic data.
51. How do you handle Kp and Kc for reactions involving ions in solution?
For reactions involving ions in solution, Kc is typically used. The concentrations of ions are used in the equilibrium constant expression. For very dilute solutions, activity coefficients might be needed to account for ion-ion interactions.
52. Can Kp and Kc be used to predict the spontaneity of a reaction?
Yes, the magnitude of Kp or Kc is related to the spontaneity of a reaction. A large K (K >> 1) indicates a spontaneous forward reaction, while a small K (K << 1) indicates the reverse reaction is spontaneous. This is directly related to the sign of ΔG°.
53. How do Kp and Kc relate to the concept of equilibrium position?
The values of Kp and Kc indicate the position of equilibrium. A large K means the equilibrium lies far to the product side, while a small K means it lies toward the reactant side. This provides information about the relative amounts of products and reactants at equilibrium.
![k_c=\frac{[c]^d[D]^d}{[A]^a[B^b]}](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650281372682.png)
![kp=\frac{[p_c]^c[p_o]^d}{[p_A]^a [p_B]^b}](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650281740782.png)
![k_c=\frac{[C]^4[D]^5}{[A]^2[B]^3}](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650282183700.png)
![k_p=\frac{[P_c]^4[p_D]^5}{[P_A]^2[P_B]^3}](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650282429373.png)
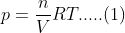
![k_p=\frac{[p_c]^c[p_D]^d}{[p_A]^a[p_B]^b}.......(2)](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650283167132.png)
![k_c=\frac{[c]^c[D]^d}{[A]^a[B]^b}.......(3)](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650283264273.png)
![k_p=\frac{[c]^c [RT]^c[D]^d [RT]^d}{ [A]^a [RT]^a[B]^b[RT]^b}](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650283798898.png)
![k_p=\frac{[c]^c [D]^d[RT]^{(c+d)}} { [A]^a [B]^b[RT]^{(a+b)}}](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650284538943.png)
![k_p=\frac{[c]^c [D]^d[RT]^{(c+d){(a+b)})}} { [A]^a [B]^b](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650285177932.png)
![k_p=\frac{[c]^c[D]^d}{[A]^a[B]^b}[RT]^{\Delta n}.........(4)](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650286002108.png)

