How Many Unit Cells are Present in a Cube-Shaped Ideal Crystal of NaCl
Introduction
An ideal NaCl crystal is a cubic-shaped crystal and is shaped as a face-centred cubic array of cations interpenetrating the fcc anion lattice. Each ion is surrounded by six other oppositely charged anions, and the cell remains the same whether we take cations or anions as corners. Each ion has a local octahedral geometry. A unit cell contains 4 molecules of NaCl. Using this information we can calculate the number of unit cells in a cube-shaped ideal crystal of NaCl of mass m.
What is NaCl
NaCl or Sodium Chloride, also known as salt, is found in the form of rock salt or can be found in oceans and seas.
Seawater contains 1%-5% of salt, and it looks like a white crystalline solid, having a molecular weight of 58.5 g/mol.
The mass of one mole of Cl is 35.5 g and the mass of one mole of Na is 23 grams, and since they are present in a 1:1 ratio, their weight amounts to 58.5 grams/mol.
Unit Cell of NaCl
A unit cell of NaCl is a Face-Centered Cubic close-packed lattice.
You can try drawing the lattice by placing the cations at the corners of the cube and at face-centred positions, and cations occupying the voids.
Thus, we can see that each sodium ion is surrounded by 6 chloride ions and each chloride ion is surrounded by 6 sodium ions, making its coordination number to be 6.
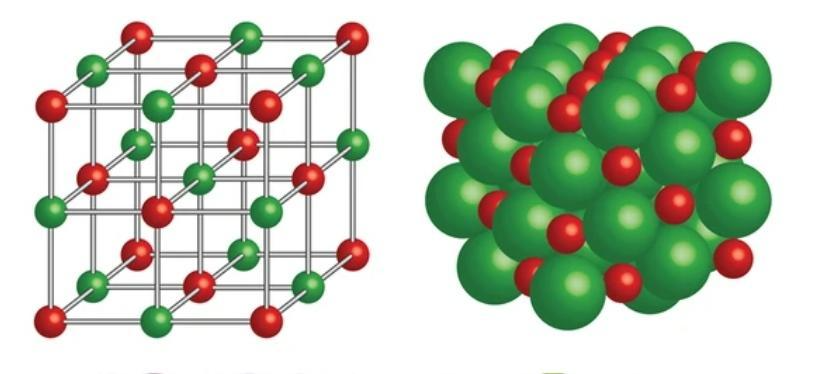
In the figure the green sphere corresponds to a bigger molecule and thus, it is a chloride ion or (Cl- ) while the red one is sodium ion or (Na+).
Mass of x Moles Unit Cells of NaCl
One unit cell has 4 NaCl molecules, and mass of one mole of NaCl is 58.5grams, thus, mass of 4 moles of NaCl i.e. mass of one moles of unit cell is ![]() .
.
Therefore, mass of x moles of unit cell of NaCl would be given by the expression ![]() , where x is the number of moles of unit cells.
, where x is the number of moles of unit cells.
Number of Unit Cells in Given Mass of NaCl
Suppose we are being given some mass of NaCl and we are told to find the Number of Unit Cells available in it, then we will do the following steps to calculate that number.
Let us start by giving an example:-
Suppose we need to find the number of unit cells in 2 grams of NaCl, then firstly we would find the number of moles of NaCl molecules in it, i.e. we would divide 2 by 58.5 to get the number of moles of NaCl which would come out to be 0.034 moles, now, since 4 moles of NaCl molecule contains one mole of unit cells in it, therefore, one mole of NaCl molecule would contain ![]() moles of unit cells which amounts to 0.25 moles of unit cells, thus,
moles of unit cells which amounts to 0.25 moles of unit cells, thus,
0.034 moles of NaCl would contain 0.034\times0.25=0.0085 ![]() moles of unit cells, and since one mole unit cell means 6.022\times{10^{23}}
moles of unit cells, and since one mole unit cell means 6.022\times{10^{23}} ![]() unit cells, thus, 0.0085 moles of unit cells would contain 0.0085\times6.022\times{10^{23}}
unit cells, thus, 0.0085 moles of unit cells would contain 0.0085\times6.022\times{10^{23}} ![]() moles of unit cells which would be 5.1187\times10^{21}
moles of unit cells which would be 5.1187\times10^{21} ![]() unit cells.
unit cells.
Generalisation of Formulas
From the above calculations we have discovered the method by which we can calculate the number of unit cells in a given mass of NaCl without knowing any generalised mathematical formula but formulas are always useful when we have got lesser amount of time, especially in the examinations.
We will be dividing the process into multiple steps so, as to make every step clear.
Let there be x mass of NaCl
Divide the given mass of NaCl by its molar weight i.e. divide x by 58.5, which would give the number of moles of NaCl present.
After that, divide the number of moles of NaCl present by 4 to get the number of moles of unit cells present.
And after that, multiply the number of moles of NaCl unit cells present by Avogadro's number to get the actual number of unit cells present in that mass of NaCl.
\frac{x\times6.022\times10^{23}}{58.5\times4} ![]() is the generalised formula where x is the mass of NaCl.
is the generalised formula where x is the mass of NaCl.
Conclusion
A unit cell is the most elementary structure of a crystal, which can be continuously repeated in order to get the bigger molecule. NaCl is glued together due to the electrostatic force of attraction, forming octahedral geometry.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
