How Many Subshells are Associated with n Equals 4
Introduction
The electrons in an atom are arranged into shells that encircle the nucleus, with each shell advancing away from the core. Atomic orbitals make up subshells, while electron shells comprise one or more subshells. While electrons in different shells or subshells have different energies, those in the same subshell share the same energy. Shells are a collection of subshells with the same principal quantum number, and subshells are a group of orbitals with the same central quantum number or angular momentum quantum number.
Definition
Simply explained, the electrons' minuscule journey within a shell is the definition of a subshell. Additionally, they are categorised from lowest to maximum energy. They are further categorised based on how much energy and electrons they can retain.
What are Shell and Subshell?
Shell
The route that electrons take to circle an atom's nucleus is known as a shell. An atom is surrounded by several shells of various energies. Around the nucleus, the shells are organised in increasing order. The shell with the lowest energy is placed first, while the shell with the highest energy is located the farthest away from the nucleus. They are known by the letters K, L, M, N, etc.
Based on its primary quantum number, each shell has a maximum number of electrons it can accommodate (n). ![]() (2n^{2}) is the formula, where n is the shell number.
(2n^{2}) is the formula, where n is the shell number.
Subshell
The quantum number of angular momentum serves as the basis for the names of the subshells. In a shell, there are four different types of subshells: s, p, d, and f. The energy of the s- subshell is the lowest, followed by that of p, d, and f. They have a certain structure as well. The "s" subshell is spherical, the "p" is shaped like a dumbbell, and the "d" is shaped like a double dumbbell.
Energy & Subshells
With the rising main quantum number, the energy of the principal quantum shells increases. For instance, n = 4 has more energy than n = 2.
The energy of the subshells rises as follows: s < p < d < f. The 3d orbital, which has somewhat more energy than the 4s orbital, is the lone exception to these limitations. The 4s orbital fills before the 3d orbital as a result.
Since every orbital in a given subshell has the same energy, they are all said to be degenerate. Example: The energy of
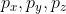 ( p_{x}, p_{y}, p_{z}) is all equal.
( p_{x}, p_{y}, p_{z}) is all equal.
Characteristics of subshells
The subshells in which electrons are distributed are determined by the azimuthal quantum number, symbolised by the letter "l."
The value of this quantum number depends on the level of the principal quantum number, n. Thus, for n = 4, four different subshells are possible.
The s, p, d, and f subshells are called following the l=0, l=1, l=2, and l=3 subshells, respectively, when n=4 is employed.
The maximum number of electrons that a subshell can hold is given by the formula
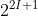 (2^{2I+1})
(2^{2I+1})The maximum number of electrons that can fit in the s, p, d, and f subshells are 2, 6, 10, and 14 respectively.
Conclusion
How many subshells are connected to the number n = 4? (given)
For a given value of "n," I can have values ranging from 0 to 1, (n-1)
So, I = 0, 1, 2, 3, 4
As a result, the four subshells s, p, d, and f are linked to n = 4.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
