How Many Sigma and Pi Bonds in Triple Bond
Introduction
A triple bond is composed of two pi bonds and one sigma bond. Covalent bonds of the types sigma and pi differ in the overlapping of atomic orbitals. A covalent bond is created when two atomic orbitals cross overlap. Bond parameters such as bond length, bond angle, and bond enthalpy are all affected by how atomic orbitals overlap. This overlap occurs in two major ways, resulting in two types of covalent bonds, namely sigma and pi bonds.
The Sigma (\sigma 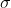 ) Bond
) Bond
Head-on positive (same phase) overlap of atomic orbitals along the internuclear axis forms this type of covalent bond. Sigma bonds are the strongest covalent bonds because the orbitals involved directly overlap. Frequently, the term "electrons" refers to the electrons in a bond. Because of the direct overlap of orbitals, sigma bonds are the strongest type of covalent bond, and the electrons in these bonds are sometimes referred to as sigma electrons.
The Pi (\pi 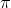 ) Bond
) Bond
Atomic orbitals parallel to the internuclear axis overlap sideways positively (same phase) to form pi bonds. When bonds are formed, the atomic orbital axes are parallel to one another, whereas when they overlap, they are perpendicular to the internuclear axis. Pi bonds are covalent chemical bonds in chemistry formed when two orbital lobes on one atom overlap with two orbital lobes on another atom, and this overlap occurs laterally. At a shared nodal plane that passes through the two bonded nuclei, each of these atomic orbitals has an electron density of zero. This plane also functions as the pi bond molecular orbital nodal. Pi bonds can form in double and triple bonds, but it does not form in single bond.
Difference Between Sigma and Pi Bond
Sigma bond | Pi bond |
During the formation of a sigma bond, during pi bond formation, overlapping orbitals must be two unhybridized orbitals. | When forming a pi bond, overlapping orbitals must be two unhybridized orbitals. |
Sigma bonds are more powerful bonds. | Pi bonds typically have lower strength than sigma bonds. |
Only one sigma bond is formed during the bonding of two given atoms. | Two pi bonds can exist between two atoms in this case. |
Sigma bonds make atoms less reactive. | Atoms with pi bonds are more reactive than those with only sigma bonds. |
Conclusion
One sigma bond and two pi bonds combine to form a triple bond. In general, sigma bonds are stronger. In molecular orbital theory, both are extensively used to predict the behaviour of molecules.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
