How Many Sigma And Pi Bonds Are In Benzene?
The strongest covalent bond created by atomic orbitals that directly overlap one another is known as the sigma bond. Alkanes, alkenes, and alkynes all contain them. A double bond is created when one sigma bond and one pi bond unite. Pi bonds are covalent chemical bonds in which two lobes from one atomic orbital from one atom are laterally overlapped by two lobes from another atomic orbital from a different atom.
Sigma and Pi bond of Benzene
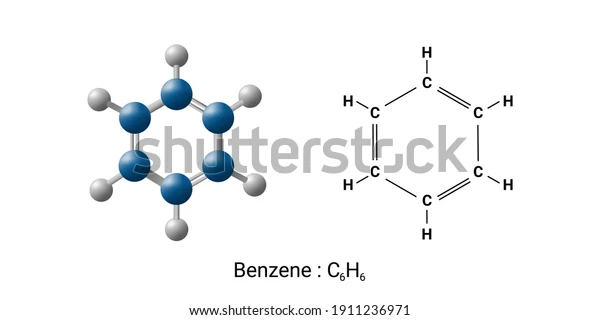
An aromatic mixture of carbon and hydrogen is benzene. The chemical name for benzene is C_6H_6
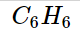
There are six C-H bonds and six C-C bonds, as can be shown.
As a result, we may state that benzene contains a total of 12 sigma bonds.
We can tell that benzene has 3 C=C bonds when we examine its structure.
Consequently, there are 3 pi bonds and 12 sigma bonds. Therefore, there are 15 covalent bonds in benzene.
One sigma bond is contained in a single bond, while one sigma and one pi bond are contained in a double bond.
Similar to this, a triple bond has two pi bonds in addition to one sigma bond.
Benzene is a substance that exists as a sweet-smelling, colourless liquid. Because the chemical is aromatic, it produces this delicious fragrance. Because of the ongoing cyclic pi bonds between the carbon atoms, benzene becomes aromatic. It is an extremely stable substance. The fundamental petrochemical benzene is found naturally in crude oil.
Benzene
The chemical compound benzene has the molecular formula C_6H_6
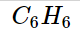 and is an organic chemical. Six carbon atoms are connected in a planar ring to form the benzene molecule, and one hydrogen atom is attached to each one. Benzene is categorised as a hydrocarbon because it solely has carbon and hydrogen atoms.
and is an organic chemical. Six carbon atoms are connected in a planar ring to form the benzene molecule, and one hydrogen atom is attached to each one. Benzene is categorised as a hydrocarbon because it solely has carbon and hydrogen atoms. One of the basic petrochemicals and a natural component of petroleum is benzene. Benzene is categorised as an aromatic hydrocarbon because of the cyclic continuous pi bonds that exist between the carbon atoms. The fragrance of gasoline is partly due to benzene, a colourless, extremely combustible chemical with a pleasant aroma. It is largely utilised as a precursor to creating chemicals with more complex structures, including cumene and ethylbenzene, which are generated in vast quantities (billions of kilos).
Occurrence
Coal and petroleum both contain traces of benzene. It results from the incomplete combustion of a variety of elements. Up until World War II, benzene was mostly produced as a byproduct (also known as "coke-oven light oil") for the steel industry. However, the synthesis of benzene from petroleum became necessary in the 1950s due to the rise in benzene demand, particularly from the expanding polymers industry. Today, the majority of benzene is produced by the petrochemical sector; only a small portion is made from coal.
Structure
The six carbon-carbon bonds in benzene are all the same length, at 140 picometers, according to X-ray diffraction (pm). C-C bonds have lengths that are longer than double bonds (135 pm) but less than single bonds (147 pm). The electrons required for C=C bonding are uniformly distributed among the six carbon atoms, which results in the intermediate distance being generated by electron delocalization. Hexane, the parent alkane of benzoene, has 14 hydrogen atoms, whereas benzoene only has 6. The only structural differences between benzene and cyclohexane are the ring of delocalized electrons and the loss of one hydrogen per carbon. Planar describes the molecule.
Characteristics Of Benzene
Below are some of the benzene's many characteristics:
Water does not mix with benzene, but organic solvents do.
It is a colourless liquid with a pleasant scent.
Its density of it is 0.87g cm-3. Compared to water, it is lighter.
Benzene has a high melting point and a moderate boiling point. (80.5°C for boiling, 5.5°C for melting)
Resonance is seen in benzo.
It burns with a sooty flame and is quite flammable.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
