How Many Quantum Numbers Are Required To Identify The Orbital?
Quantum numbers are defined as they are used to describe the trajectory and the movement of any electron in an atom and all the electrons in a given atom, when combined together they must comply with the Schrodinger equation.
This Story also Contains
- Introduction
- Quantum Numbers and Atomic Orbitals
- Electron Spin Quantum Number
- Main Content
Introduction
The set of numbers which is used to describe the position and energy of the electron in an atom is called quantum numbers. There are a total four quantum numbers, They are
Principal
Azimuthal
Magnetic
Spin quantum numbers.
The values of the conserved quantities of a quantum system are always given by quantum numbers and electronic quantum numbers is the quantum numbers which describes the electrons and can be defined as the group of numerical values that can be provide solutions which is acceptable by the Schrodinger wave equation for hydrogen atoms.
Quantum Numbers and Atomic Orbitals
Four quantum numbers is used to completely describe all the attributes of a given electron which belongs to an atom, these are given below:
Principal quantum number is denoted by n.
Orbital angular momentum or it can be said as azimuthal quantum number is mostly denoted by l.
Magnetic quantum number is denoted by m_{l}
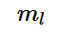 .
.The electron spin quantum number is denoted by m_{s}
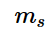 .
.
Principal Quantum Number
Principal quantum numbers are denoted by the symbol ‘n’ and they designate the principal electron shell of the atom. The distance between the nucleus and the electrons is described as the larger value of the principal quantum number which implies a greater distance between the electron and the nucleus which implies a great atomic size.
The value of the principal quantum number may be any integer number with a positive value and that can be equal to or greater than one(1). The value when n=1 , it belongs to the innermost electron shell of an atom, which only corresponds to the lowest energy state or to the ground state of an electron.
Therefore, it can be understood that the principal quantum number which is denoted by n it cannot have a negative value or be equal to zero, the reason behind is that the atom can’t have a negative value or no value for a principal shell.
When a given electron is infused with energy that is in an excited state, it can be observed that the electron jumps from one principal shell to the higher shell which causes an increase in the value of n. When electrons lose energy, they jump back into lower shells and the value of n will decrease.
Absorption is defined as the increase in the value of n for an electron when emphasizing the photons of energy being absorbed by the electron and the decrease in the value of n for an electron is known as emission, where the electrons emit their energy.
Azimuthal Quantum Number (Orbital Angular Momentum Quantum Number)
The azimuthal or it can be said as orbital angular momentum the quantum number describes the shape of a given orbital and it is also denoted by the symbol ‘l’ and its value is equal to the total number of angular nodes in the orbital.
A value of the azimuthal quantum number can indicate either an s, p, d, or f subshell which may vary in shape. This value depends on the value of the principal quantum number that is the value of the azimuthal quantum number that ranges between 0 and (n-1).
Magnetic Quantum Number
Magnetic quantum number is defined as the total number of orbitals in a subshell. Magnetic quantum number is denoted by the symbol m_{l} ![]() and this number yields the projection of the angular momentum which corresponds to the orbital with a given axis.
and this number yields the projection of the angular momentum which corresponds to the orbital with a given axis.
Shapes of Orbitals
The value of the magnetic quantum number is dependent on the value of the azimuthal or to the orbital angular momentum. For any given value of l, the value of m_{l} ![]() ranges between the interval -l to +l. So this means it indirectly depends on the value of n.
ranges between the interval -l to +l. So this means it indirectly depends on the value of n.
Electron Spin Quantum Number
The electron spin quantum number is always independent of any values of n, l, and m_{l}
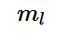 and the value of this electron spin quantum number gives insight to the direction in which the electron is always spinning, and denoted by the symbol m_{s}
and the value of this electron spin quantum number gives insight to the direction in which the electron is always spinning, and denoted by the symbol m_{s} 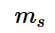 .
.The value of m_{s}
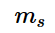 offers insight into the direction in which the electron is spinning and the possible values of the electron spin quantum number is +½ and -½.
offers insight into the direction in which the electron is spinning and the possible values of the electron spin quantum number is +½ and -½.The positive value of m_{s}
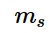 is an upward spin on the electron and it is also known by ‘spin up’ and is denoted by the symbol \uparrow
is an upward spin on the electron and it is also known by ‘spin up’ and is denoted by the symbol \uparrow 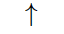 . If m_{s}
. If m_{s} 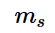 has a negative value,if the electron in question is said to have any downward spin, or also known by ‘spin down’, which is given by the symbol \downarrow
has a negative value,if the electron in question is said to have any downward spin, or also known by ‘spin down’, which is given by the symbol \downarrow  .
.The value of the electron spin is determined whether the atom in question has the ability to produce any magnetic field and the value of ms can be be generalized to ±½.
Summary
Name and Symbol | Meaning and Possible Values |
Principal quantum number, n | Electron shell, n ≥ 1 |
Azimuthal quantum number, l | Subshells (s=0, p=1, etc.) , (n-1) ≥ l ≥ 0 |
Magnetic quantum number, ml | Total number and orientation of orbitals, l≥ml≥-l |
Electron spin quantum number, ms | The direction of electron spin, ms = ±½ |
Main Content
The quantum number represented by n is the principal quantum number, which indirectly specifies the size of the electron orbital and it is always given an integer number, but it can never be equal to zero which describes the energy level.
The azimuthal quantum number is represented by “l” and its value is 0,1,2,3....(n-1)
Magnetic quantum number divides subshells into distinct orbitals and places the electron in one of them and it is represented by “m” and its value can be negative, positive, or can be equal to zero.Magnetic quantum number specifies the direction of an orbital of given energy (n) and shape (I).
Spin quantum number describes the spin of the electron and it is represented by “s' ' or ms and its value is -12or+12.
Therefore, four quantum numbers are required to identify the given orbital.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
