How Many Moles of Electron Weighs One Kilogram
Introduction:
Approximately 1.82 × 106 moles of electrons will weigh one kilogram.We know that an electron has a negative charge and it can be either bound with an atom or it can exist in the form of a free charge also. A kilogram is a unit of mass. In this article, we will learn about how many moles of electrons will be there in one kilogram of weight. But before discussing the actual subject, let's briefly know the details of electrons, moles, and kilograms. Also, we will be discussing frequently asked questions related to this topic in this article.
Define Electron:
The electron was discovered by J. J. Thomson in 1897. Electron simply means a negatively charged particle present in every atom. It is either bound in an atom or free. When the number of electrons equals the number of protons, we get a neutral atom.
Charge of an electron = 1.6 × 10-19 C.
Define Mole:
We can simply define mole as the number used for measuring the amount of any given substance, which is equal to 6.02214 × 1023. Also, mol is the symbol used for mole.
Step By Step Calculation-
We know the mass of an electron = 9.1 × 10-31 kg.
\because 9.108 \times 10^{-31} \mathrm{~kg}=1.0 \text { Electron }
![]()
1 \mathrm{~kg}=\frac{1}{9.108 \times 10^{-31}}electrons
![]()
We know 6.023 × 1023 electrons make 1 mole, hence
\\ \ \frac{1}{9.108 \times 10^{-31}}\text { Electrons }=\frac{1}{9.108 \times 10^{-31}} \times \frac{1}{6.023 \times 10^{23}} \text { moles of Electrons }\\ \ \Rightarrow \frac{1}{9.108 \times 10^{-31}}\text { Electrons }=\frac{1}{9.108 \times 6.023} \times 10^8 \text { moles of Electrons }
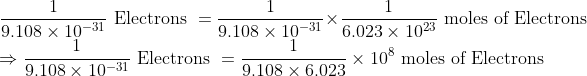
So approximately 1.82 × 106 moles of electrons will weigh one kilogram.
Notes:
The approximate mass of an electron is 9.1 × 10-31 kg.
Also, it's negligible in comparison to the mass of protons or mass neutrons. Therefore the mass of electrons is not used in calculating the mass number of atoms.
1-mole electron is equal to 6.022×1023 electrons, where 6.022×1023 is an Avogadro number.
The charge on an electron is 1.6 × 10-19 C, and the mass of an electron is 9.1 × 10-31 kg.
Examples
How Many Moles of Electron Weighs 2 Kilogram?
The weight of a single mole of electrons is equal to the atomic mass of an electron, which is approximately 9.109 \times 10^{31} ![]() kilograms. To find out how many moles of electrons weigh 2 kilograms, you can use the formula:
kilograms. To find out how many moles of electrons weigh 2 kilograms, you can use the formula:
Number of moles = weight (in kilograms) / molar mass (in kilograms per mole)
In this case, the molar mass of electrons is 9.109 \times 10^{31} kg/mol ![]()
So,
Number of moles = 2 / 9.109 \times 10^{31} ![]()
This results in approximately 2.19 \times 10^{30} ![]() moles of electrons.
moles of electrons.
How Many Moles of Electron Weighs 3 Kilogram?
The weight of a single mole of electrons is equal to the atomic mass of an electron, which is approximately 9.109 \times 10^{31} ![]() kilograms. To find out how many moles of electrons weigh 2 kilograms, you can use the formula:
kilograms. To find out how many moles of electrons weigh 2 kilograms, you can use the formula:
Number of moles = weight (in kilograms) / molar mass (in kilograms per mole)
In this case, the molar mass of electrons is 9.109 \times 10^{31} kg/mol ![]()
So,
Number of moles = 3 / 9.109 \times 10^{31} ![]()
This results in approximately 3.28 \times 10^{30} ![]() moles of electrons.
moles of electrons.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
