How Many Moles are There in The 34.5 g of Sodium(Na)
There are 1.5 moles in 34.5 g of Sodium(Na). To find the number of moles, one has to find the molar mass of one molecule of the given compound or element. Then the number of moles can be calculated by dividing the given mass of the element or compound by its molar mass. In the given question, we are required to find the number of moles in 34.5 Grams of Sodium. In the following method, the process of finding the number of moles is explained.
Mass of the element Sodium = 34.5 grams
Molar mass = 23 grams
Total moles = 34.5/23 = 1.5 moles
Mole(mol)
A mole is a concept that relates the masses of substances to the chemical equations involved. It is the amount of a substance that contains as many entities(atom, ion, molecule) as there are in 12 Grams of C-12 Carbon isotope.
6.022 \times 10^{23}
One mole equals atoms or molecules of the substance given. ![]() is also known as Avogadro’s Constant.
is also known as Avogadro’s Constant.
One mole is equal to the mass of the substance given divided by the molar mass of the molecule of that same substance.
One mole can also be equal to the number of atoms, ions or molecules provided divided by Avogadro’s Constant.
For example, now we need to find the number of moles in 180 grams of water.
The molar mass of H(Hydrogen) is 1, and Oxygen is 16. So the Total molar mass of 1 molecule of water is 18.
the number of moles = 180/18 = 10. The answer is 10 moles.
The SI Unit of a substantial amount is Mole.
Molar Mass(M)
The Molar mass is also known as molecular weight, and it is the mass associated with one mole. Each element present on the periodic table has an atomic mass, which is the approximate mass of a single atom.
For example, if we need to find the molar mass of Sulphuric acid (H2SO4), we will find the weighted sum of each elemental molar mass.
(\underbrace{2 \times 1.01}_{\text {mass of } \mathrm{H}}+\underbrace{32.06}_{\text {mass of S }}+4 \times \underbrace{16.00}_{\text {mass of } O}) \bullet g \bullet \mathrm{mol}^{-1}=\underbrace{98.08 \bullet g \bullet \mathrm{mol}^{-1}}_{\text {molar mass of sulfuric acid }}
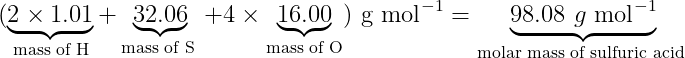
The most commonly used unit of molar mass is Gram per unit mole, although its SI unit is kg/ mol.
Without the molar mass, it would be impossible to solve the chemical reactions.
Conclusion
When we think of the amount of a material or substance, we think about its weight or the volume it's going to occupy. However, as we know, the densities of different elements or compounds are different, and volume and weight should not be the only measures. A small-sized object made up of iron can weigh way more than a big-sized object of Plastic. Hence to meet the requirements of the scientific calculations, we have made the “mole” a measure of the substance amount. The molar mass of a substance is relatively defined by the mole. Chemists measure using moles for very small entities like atoms, molecules, or other particles.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
