How Many Isomers of Propane are There
Introduction
Propane or C3H8 has no isomers. Isomers are two or more compounds with the same formula but different spatial arrangements. Propane has 3 carbon atoms and 8 hydrogen atoms, making it practically impossible for the compound to have any other arrangement. In order to understand the reason behind it, one must first understand the structure of propane.
Structure of Propane
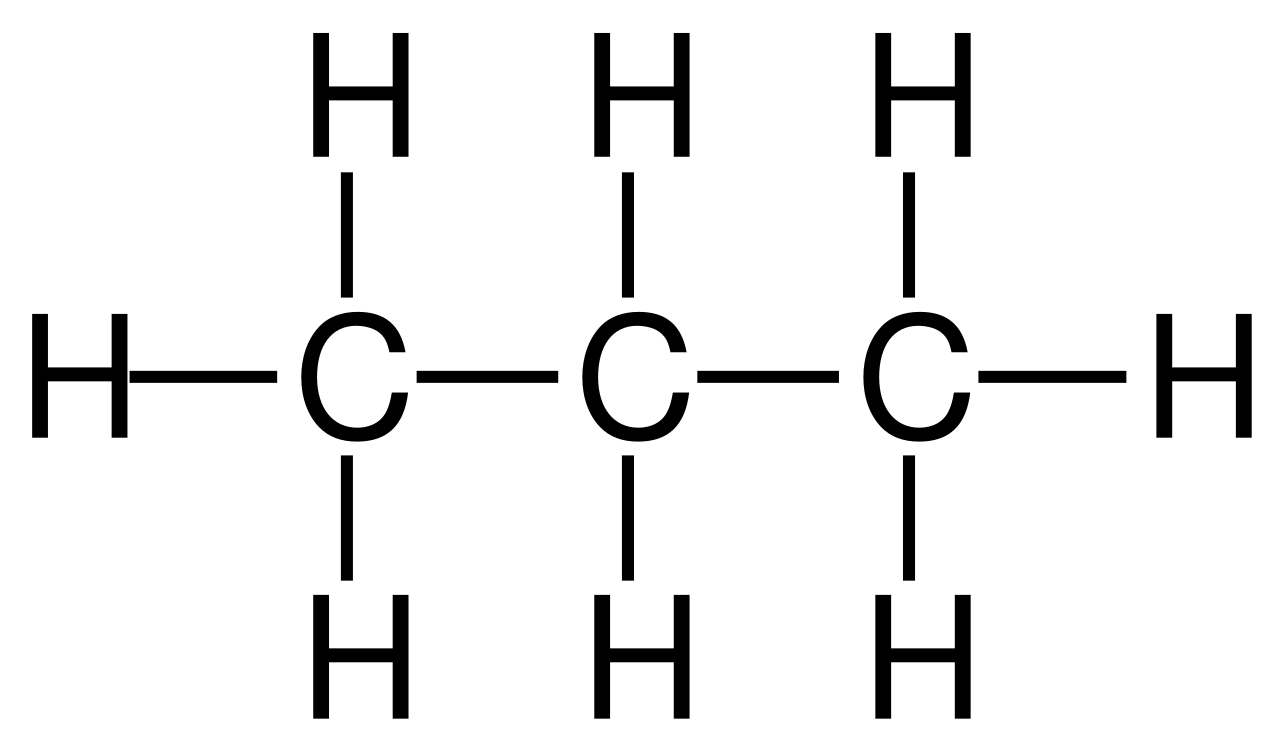
Propane has the formula C3H8 and shows a chained alkali structure. This implies that the compound is made up of 3 atoms of carbon and 8 atoms of hydrogen. The three carbon atoms are arranged in a line with hydrogen atoms attached to them in space.
Isomerism
Isomerism is the phenomenon by which a compound can exist in two or more forms. It is of two types -
Structural isomerism - This isomerism occurs when a compound has a close relative which differs merely in its structure from the original compound but still shares the same formula. Eg. 1-Chloropropane and 2-Chloropropane.
Stereo isomerism - This isomerism occurs when a compound has the same formula as another compound but the atoms are arranged differently in both compounds. Eg. cis-but-2-ene and trans-but-2-ene.
Isomer of Propane
Propane is composed of only two atoms - carbon and hydrogen. In any compound, carbon forms two bonds and hydrogen forms one. The first carbon in propane is attached to three hydrogen atoms and a second carbon atom. This second carbon atom is attached to the first carbon atom and a third carbon atom along with two hydrogen atoms. The third carbon atom in propane is attached to the second carbon atom by a bond and to three hydrogen atoms.
Since the whole structure of propane is uniform, it leaves no space for isomerism to occur which needs at least more than three carbon atoms or three kinds of atoms to form a new structure.
Uses of Propane
Propane is used in the following places -
As a refrigerant
As fuel in hot-air balloons
As fuel in ships
For drying clothes
In farm and industrial equipment
In blowtorches used for soldering
As fuel for cooking
The liquid form of propane is used for the extraction of vegetable oil and animal fat.
Conclusion
With the composition of three carbon atoms and eight hydrogen atoms, propane is incapable of forming an isomer. The making of an isomer requires a compound to have at least more than three carbon atoms or an atom which can break the symmetry of the compound. Since none of the conditions gets fulfilled in the case of propane, the compound is left with only one structure and no isomers.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
