How Many Isomers of C5H11OH Will Be Primary Alcohols
Introduction
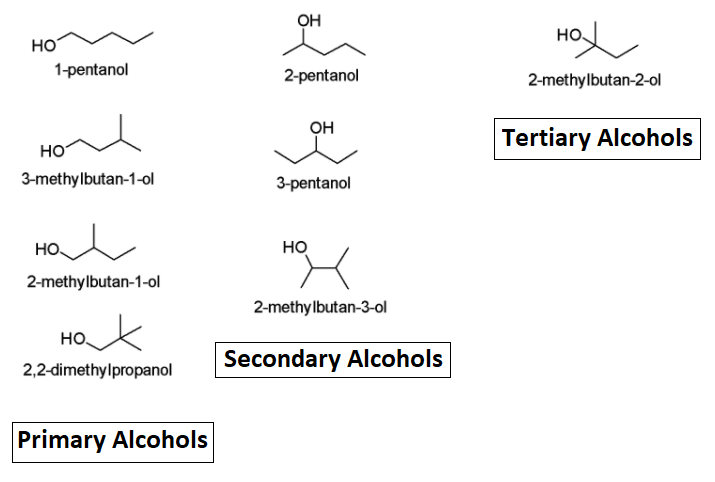
The number of isomers of \mathrm{C}_5 \mathrm{H}_{11} \mathrm{OH}![]() is 4(four) will be the primary alcohol. To understand how and why let us proceed towards another topic section.
is 4(four) will be the primary alcohol. To understand how and why let us proceed towards another topic section.
Brief Description
First, let us understand what primary alcohols are and their total types. So, primary alcohol is the alcohol with the hydroxyl group bonded to a primary carbon atom. It can also be referred to as a molecule containing -\mathrm{CH}_2 \mathrm{OH}“![]() ”.
”.
Let us proceed with the understanding of Isomers now. They are compounds that contain precisely a similar number of atoms; in other words, they have the same verifiable formula, but they differ from each other in the way the atoms are arranged.
There are three types of Isomers which are “Constitutional Isomers, Stereoisomers, Enantiomers, And Diastereomers. It is a fact that they’re all related. One key difference between families and molecules is at a point in time, an individual can be a father and a brother. However, in the organic chemistry family structure, there are no such problems.
There are two molecules that might be stereoisomers of each other; however, they can never be stereoisomers and constitutional isomers of each other. In such a manner, their differences are made very clear.
Now, how do we differentiate a pair of Non-Isomers from a couple of Isomers:
Isomers are two or more molecules which are said to share the same molecular formula.
Conclusion
So, now at the end, let us understand how the types of Isomers are different from each other. Constitutional isomers have indistinguishable molecular formulas but varied connectivities. Stereoisomers have similar connectivity, but they have different arrangements of their atoms in space. Diastereomers are the stereoisomers which are not non-superimposable mirror images. Enantiomers are those stereoisomers which are non-superimposable mirror images.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
