How Many Groups and Periods are There in The Mendeleev Periodic Table
Introduction
The sixty-three elements in Mendeleev's periodic table are organised in increasing order of atomic mass. There are eight groups and seven periods on the table. The periodic table is a collection of chemical elements that are tabulated and sorted by atomic number, from hydrogen through oganesson, the element with the highest atomic number. The quantity of protons in the nucleus of an element's atom is known as the element's atomic number. Oganesson possesses 118 protons compared to hydrogen one.
Dmitri Mendeleev and Mendeleev Table
The Russian physicist Dmitri Ivanovich Mendeléev made the most significant contribution to the early construction of the periodic table. The Mendeleev periodic table was the most significant of all the periodic tables created. After the Newlands Octave Law was rejected in 1869, the Mendeleev Periodic Table entered the scene. In Mendeleev's periodic table, elements were arranged according to their fundamental property, atomic mass, and chemical characteristics. Only 63 elements were understood at the time of Mendeleev's work. Mendeleev discovered a periodic relationship between the qualities of elements and atomic mass after researching the characteristics of each element. He organised the elements so that they fell into the same vertical columns of the periodic table and had similar qualities.
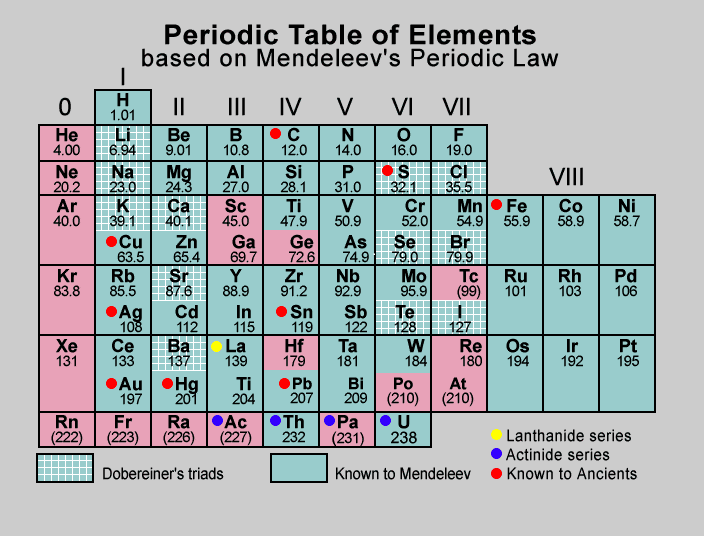
Explanation
The periodic table lists elements in increasing order of their respective atomic masses.
Eight groups and seven periods make up Mendeleev's periodic table.
Groups I through VII are for normal elements, and group VIII is for elements in transition.
While groups VIII are intended for three elements, groups I through VII have been broken into two subgroups.
The fourth through seventh grades have been split into the first and second series.
Elements with related properties have been grouped. Lithium, potassium, rubidium, etc., are examples of first-group elements.
The elements in each group of the periodic table have been given two general equations, one for oxides and the other for hydrides. For instance, the first group of elements' oxides and hydrides are R2O and RH, respectively. The oxides and hydrides formulas for the elements in each group can be written using the general formulae provided. For instance, the first category includes hydrogen, sodium, potassium, and other elements. R2O is the formula of the universal oxide for the first group of elements. As a result, they create H2O, Na2O, K2O, etc. Mendeleev occasionally had to position an element with a slightly higher atomic mass ahead of an element with a slightly lower atomic mass while creating the periodic table.
Additionally, Mendeleev's periodic chart has several gaps. Mendeleev boldly predicted the presence of some elements that weren't yet known, refusing to view these gaps as flaws. Mendeleev gave them names by appending an Eka (one) to the name of the element in the group before it. For instance, it later found elements like scandium, gallium, and germanium share characteristics with eka-boron, eka-aluminium, and eka-silicon, respectively. Before this, numerous instances had been made of noble gases, including helium, neon, and argon.
Limitations of Mendeleev's periodic table: When hydrogen and alkali metals react with halogens, oxygen, and sulphur, they produce a compound with a similar formula. As a result, the electronic configuration of hydrogen is similar to that of alkali metals. The initial drawback of Mendeleev's periodic table was that hydrogen could not be assigned a definite location.
Conclusion
In Mendeleev's periodic table, the 63 elements are arranged in increasing order of atomic mass. On the table, there are seven periods and eight groups. The periodic table is a collection of chemical elements that are tabulated and sorted by atomic number, from hydrogen through oganesson, the element with the highest atomic number. The Russian physicist Dmitri Ivanovich Mendeléev made the most significant contribution to the early construction of the periodic table. In Mendeleev's periodic table, elements were organised by their fundamental properties, atomic masses, and chemical properties. According to their atomic masses, elements are listed in the periodic table in increasing order.
One of many important discoveries was the Mendeleev periodic table. The periodic table created by Mendeleev has eight groups and seven periods. Two generic equations, one for oxides and the other for hydrides, have been given for each group of the periodic table's elements. The generalised oxides formula for first group elements is R2O. Mendeleev's periodic table also features several gaps. Mendeleev's periodic table has some drawbacks, including the formation of compounds with similar formulas when halogens, oxygen, and sulphur are combined with hydrogen and alkali metals.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
