How Many Grams of Dibasic Acid Mol wt. 200 Should be Present in 100 ml of an Aqueous Solution to Give
Introduction
Dibasic acid of 1 gram should be present, Mol Weight 200, should be there in 100 ml of the aqueous solution, which gives the decinormal strength. Decinormal strength is an adjective used in chemistry, a solution where it contains 0.1, the equivalent weight per solute in the solution. Aqueous solutions are solutions in which the solvent is water.
Let Us Understand This in Brief
To understand the entire composition mentioned above, let us understand the terminologies and then the formulas.
Dibasic Acid acid is a compound containing Hydrogen ions, denoted by the H+ sign. This acid is easily replaceable with a metal or a basic radical. The formulae for the same are;
\begin{aligned}
& \mathrm{N}=\frac{\text { Givenweight }}{\text { Equivalent weight }} \times \frac{1}{\text {volume of solution (L)}} \\
& \mathrm{N}=0.1 \mathrm{~N} \\
& \text {Molecular weight }=200 \\
& \text {Equivalent weight }=\frac{200}{\text { Basicity }}=\frac{200}{2}=100 \\
& \mathrm{~V}=100 \times 10^{-3} \mathrm{~L}=0.1 \\
& \textup{ So, } 0.1=\frac{x}{100} \times \frac{1}{0.1}
\end{aligned}

This particular acid produces two Hydrogen ions which are known as dibasic acid. It segregates into two steps. Therefore, its basicity is 2. To understand, for example, Sulfuric acid, Carbonic acid, and Oxalic acid, where sulfuric acid has two hydrogen ions and is known as dibasic acid.
Along with it, there are a few other concepts that need to be taken care of while understanding the relations between these acids, namely, “Aqua fortis,” “Aqua regia,” “Fuming nitric acid,” and “Decrepitation.”
Let us now move forward toward these chemicals:
Aqua fortis is nothing but the ancient terminology of nitric acid.
Whereas, Aqua regia is a combination of concentrated nitric acid and concentrated hydrochloric acid. Mix in a 1:3 ratio for an ideal combination. Aqua regia can dissolve precious metals. To understand the volume ratio of nitric acid to hydrochloric acid in aqua regia means that it is a 3:1 mixture of hydrochloric acid and nitric acid. That is, a ratio of 3 parts hydrochloric acid to 1 part nitric acid.
When nitric acid is mixed with nitrogen dioxide, then it forms Fuming nitric acid. It is procured from refining nitric acid through a small amount of starch. This starch is broken down into nitric acid to nitrogen dioxide, which is dissolved in the leftover nitric acid. The remaining nitric acid in this process forms fuming nitric acid, which is turned into a brownish colour.
When chemicals such as lead nitrate are heated, there is a cracking sound produced, and decrepitation is the decomposition of that substance.
Conclusion
After understanding all the above concepts, it is clear how the decinormal strength is formed. Therefore, to derive the dibasic acid, the actual formula is as follows;
E=\frac{M}{2}=\frac{200}{2}=100
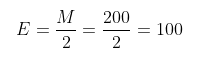
\begin{aligned}
\mathrm{N} & \mathrm{=\frac{W \times 1000}{E \times V(\text { in ml })}} \\
\mathrm{\frac{1}{10} }& =\mathrm{\frac{W \times 1000}{100 \times 100}
}\\ \textup{W} & \textup{= 1 \ gm}
\end{aligned}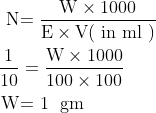
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
