How Many Geometrical Isomers are Possible for The Following Compound
Introduction
Geometrical isomers are those chemical compounds (i.e. organic or inorganic compounds) that have the same number and kind of atoms, the same type of bonding or connectivity but different spatial arrangements. Isomers are those chemical compounds which have the same empirical formula but different structural formula. This type of isomerism is mainly found around the double-bonded carbon atoms or ring structure. It shows the orientation or position of the functional group present in the compound.
On the basis of the position of functional groups, the compound is named in two ways-
Cis
Trans
Cis isomers are those compounds in which the functional groups are present on the same side of the double bond. It is also represented by "E".
Trans isomers are those which have functional groups on the opposite of double bond. It is represented by "Z".
The names of these compounds have prefixes cis and trans accordingly.
Cis-trans isomerism is also known as E-Z isomerism.
What is an Isomer?
Isomers are those complexes that have the same molecular formula but different structural formulas. It is not necessary that the isomers have similar chemical and physical properties. Isomerism is found in compounds which have the existence of isomers in them.
There are mainly two types of isomerism present in chemical compounds-
Structural isomerism
Stereo isomerism
And, further, these are also divided into subcategories.
How to Find Out Geometrical Isomerism in Any Chemical Compound?
First of all, we just need to find double-bonded carbons which are connected to the different types of functional groups.
Next, we know that two types of isomers cis and trans are possible in this case.
So, two geometrical isomers around one such double bond.
We have to find all such double-bonded carbons and calculate the total no. of geometrical isomers.
Let us see it with an example-
C6H8
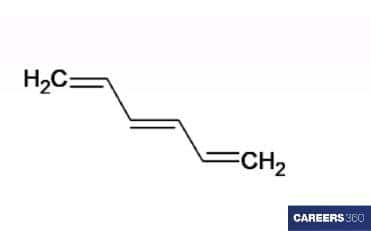
By the structure of this compound, we found that only one middle double bond is present in which the same type of functional groups are connected. So, only two isomers that is, cis and trans isomers are possible in this organic compound.
but-2-ene
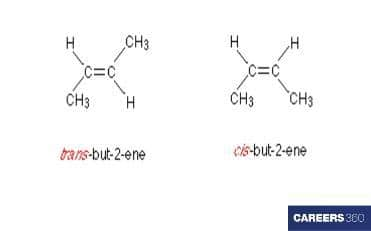
There is only one double bond in this organic compound around which two isomers are possible cis and trans. So, total geometrical isomers are two in this organic compound.
Now, let's see an example of inorganic compounds.
[Pt(Cl)2(NH3)2]
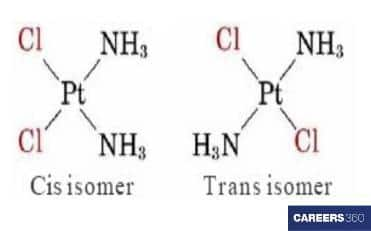
In these inorganic compounds, we check the spatial arrangement of ligands. In this compound, if Cl is on the same side of a double bond then a cis isomerism is present and if Cl is on the opposite side then it is trans isomerism. So, this compound is also having two geometrical isomers. This type of isomerism is found in heteroleptic complexes due to the different structural arrangements of ligands.
Geometrical isomerism is mostly found in square planar and octahedral complexes in inorganic compounds and several organic compounds also.
Conclusion
Geometrical isomerism is a type of stereoisomerism which is found in both organic and inorganic compounds. It comes under the subcategory of diastereomers It is important to know the type of isomerism in a compound to know the orientation or position of a functional group and find the basic structure of any compound. Through this article, we have learnt how to find the geometrical isomerism in compounds to get in-depth of the structure of the chemical compound.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
