How Many Electrons Can Go in the Third Shell
Introduction
The third shell of an atom can accommodate up to 18 electrons. These electrons get divided into s, p, d, or f subshells according to their increasing energy. But, how do we know when an electron will enter the third shell, and even if it does, which subshell it will go into? In order to understand this, one must first know about the structure of an atom.
Structure of an Atom
The structure of an atom or atomic structure is a description of the arrangement of subatomic particles inside an atom. An atom is made up of a positive centred nucleus with negatively-charged electrons revolving around it. This nucleus contains two types of subatomic particles - positively-charged protons and neutral neutrons.
Prior to this accepted model of an atom, various theories were given to understand its structure. These theories were-
Dalton’s Atomic Theory
Thomson's Atomic Model
Rutherford's Atomic Model
Bohr’s Atomic Theory
Dalton’s Atomic Model
Proposed by John Dalton, this was the first model to suggest that matter is made up of tiny indivisible particles called atoms. These atoms can neither be created nor destroyed, but atoms from different elements can be combined together in whole-number ratios to create new compounds. Dalton also asserted that atoms of different elements are different in size, mass, and properties while the atoms of the same element are identical in their sizes, masses, and properties. Although this was a revolutionary model where someone tried to understand the atomic world for the first time, this model had its own shortcomings.
Dalton viewed atoms as indivisible which negated the existence of subatomic particles.
Dalton didn't take into account the existence of isotopes.
Dalton couldn't explain why allotropes of the same element had different properties.
Atoms do not always combine in whole-number ratios to form a compound.
Thomson Atomic Model
Also known as the plum pudding model, this model is given by J. J. Thomson, suggests that the subatomic particles inside an atom are arranged in a plum pudding-like orientation. That is to say, the atom has a positively charged central sphere in which the negatively charged electrons are embedded. This model can also be compared to a watermelon, where the red juicy part is the positively charged centre and the seeds in it are the negatively charged electrons.
Despite Thomson’s efforts, this model fails on the following fronts:
Thomson was unable to explain how the positively charged centre holds the negatively charged electrons in it.
There was no experimental proof of this model.
Thomson’s atomic model couldn't explain how alpha particles scattered through a thin metal foil.
Rutherford Atomic Model
When the above atomic models failed, Ernest Rutherford tried an experiment. He bombarded a thin sheet of gold with alpha particles and recorded his observations. He observed that when he directed alpha particles at the sheet, some particles showed great deflection, some showed a small deflection, and some particles didn't deflect at all from their trajectory.
This led Rutherford to make the following observations:
Since a lot of particles didn't deflect from their original tract, there is a lot of empty space in the atom.
Since some particles showed a small deflection from their tract, he deduced that the positive charge is not uniformly distributed within an atom.
Since some particles showed great deflection, as much as returning right back to the source, Rutherford assumed that the positively charged particles must be concentrated in a small space.
Based on these observations, Rutherford gave his model of an atom -
The atom consists of positively charged protons which are concentrated in a small centre with negatively charged electrons revolving at a high speed around them in an orbit. These electrons are held together by the proton by strong electrostatic forces. Despite the experimental proof, the Rutherford Atomic Model failed to explain the stability of an atom and the arrangement of electrons inside an atom.
Bohr's Atomic Theory
Given in 1915 by Neil Bohr, Bohr’s atomic model improved upon the Rutherford atomic model. The model postulates that an atom is made up of a positively charged centre with negatively charged electrons revolving around it. These electrons do not roam around freely but move in specific orbitals around the centre. These orbitals have fixed energies and when an electron has to go up an orbital, it takes in energy, while when it has to go a lower orbital, it gives out energy equivalent to the gap between the energy levels of the two orbitals.
Amongst all the given models, Bohr’s atomic model is the most accepted, yet it has its own shortcomings -
It fails to explain the Zeeman effect.
It fails to explain the Stark effect.
It goes against the Heisenberg Principle of Uncertainty.
It fails to explain the spectra obtained from larger elements.
How Do Electrons Arrange Themselves Inside an Atom?
Although Bohr’s atomic model has its own limitations, we will be using it to explain the arrangement of electrons in an atom, as it is the most accepted model. According to Bohr’s model, the positively charged nucleus in an atom is surrounded by several shells, namely, K, L, M, N, and so on. These shells are divided into subshells (s, p, d, f) which are further divided into orbitals. The more the atomic number of an element, the more shells and subshells it has.
Shell number | 1 | 2 | 3 | 4 |
Subshell | s | s, p | s, p, d | s, p, d, f |
No. of Orbitals | 1 | 1, 3 | 1, 3, 5 | 1, 3, 5, 7 |
An electron always aspires to join the least energy orbital available to it. Therefore, it will first fill 1s, then 2s, then 2p, and so on. It is important to know that no electron occupies an orbital alone. It is always a pair of opposite spin electrons which occupy an orbital.
Shell Number | 1 | 2 | 3 | 4 |
Subshell | s | s,p | s,p,d | s,p,d,f |
No. of Orbitals | 1 | 1,3 | 1,3,5 | 1,3,5,7 |
No. of Electrons in Orbitals | 2 | 2,6 | 2,6,10 | 2,6,10,14 |
Total No. of Electrons in Subshell | 2 | 8 | 18 | 32 |
Therefore, when it comes to the electronic distribution of electrons in an atom, it goes as follows -
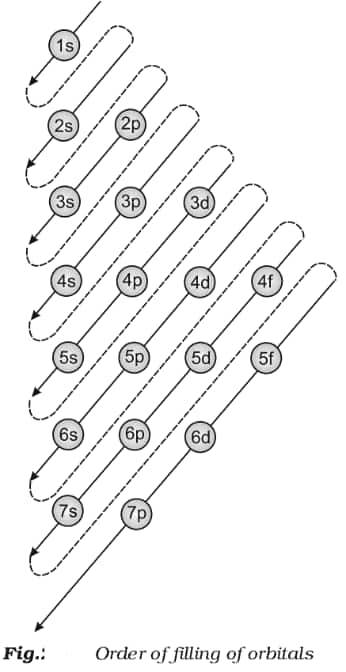
Electrons always occupy the lowest energy orbital available to them.
Two electrons of opposite spins (+ and -) occupy a single orbital.
If two orbitals share the same energy level, both of them are half-filled before a second electron joins one of the orbitals to fully fill them.
How to Write an Electronic Configuration?
Electronic configuration is the description of the arrangement of electrons in an element. While writing the electronic configuration of an element, we first write the first shell number to be filled, followed by the subshell designation and the number of electrons contained in the subshell as a superscript. For example, Potassium (K) has 19 as its atomic number. Therefore, its electronic configuration will be written as 1s2 2s2 2p6 3s2 3p6 4s1.
Conclusion
An atom is a minuscule positively charged centre with negatively charged electrons revolving around it. These electrons are equivalent in number to the atomic number of an element. They revolve around the nucleus in various shells, subshells, and orbitals according to their energy levels. Each orbital is occupied by two electrons of opposite spins, thereby owing to the fact that the third shell or M shell of any element is capable of holding a total of 18 electrons. When presented in the form of an electronic configuration, these electrons are presented in the order of the shells and subshells they fill in followed by a superscript of the number of electrons in the subshell.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
