How Many Covalent Bonds Does Ethane Have
Introduction
The chemical formula for ethane is C2H6. It is a paraffin series hydrocarbon with colourless, odourless, and gaseous properties. Ethane is the only hydrocarbon with a single carbon-carbon bond and the most uncomplicated structural makeup. The massive ethylene petrochemical sector, which creates essential goods like polyethylene plastic, ethylene glycol, and ethyl alcohol, heavily relies on ethane as a raw ingredient.
Two atoms exchange one or more pairs of electrons to create a covalent bond. These electrons are being drawn to the two atomic nuclei at the same time. A covalent bond is created when the electronegativities of two atoms are too small for an electron transfer to happen and produce ions. The electrons that are present between the two nuclei are collectively referred to as bonding electrons. The bound pair serves as the "glue" that binds the atoms in molecular units.
Explanation
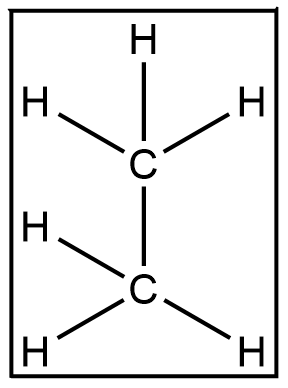
This is the simplest structure of ethane. It constitutes 2 carbon atoms and 6 hydrogen atoms.
Carbon has tetravalency i.e it can form 4 bonds. Carbon has 4 electrons in its outermost shell and in order for it to gain stability it has to follow the octet rule. The octet rule states that each atom must complete 8 electrons in its outermost shell to achieve stability.
Each of the 2 Carbons forms 4 bonds. 1 with the other carbon and 3 with hydrogen each. Each hydrogen has 1 electron in its outermost shell and requires 1 more to acquire stability. So if we look at 1 carbon at a time. The carbon shares its 4 electrons at the same time it acquires 4 electrons. This is done by sharing electrons amongst the atoms. This way the carbon does not lose any electrons rather it is able to complete its octet simultaneously assisting hydrogen and the other carbon in attaining their octets too.
All the seven bonds formed here are a result of sharing each other's electrons. This sharing of electrons to form bonds is what is referred to as a covalent bond. Hence 7 covalent bonds are contained in an ethane system.
Conclusion
Hence by looking at the structure it can be determined that each atom contained in the structure does not have much variance in its electronegativity hence they are most liable to form covalent bonds only. So every bond contained in the ethane is a covalent bond. Since 7 bonds are present in the system, it is safe to say that ethane has 7 covalent bonds.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
