How Many Conformations Does Ethane Have
Introduction
Ethane has an infinite number of conformations. Conformations are the various spatial configurations of atoms or groups that can be changed into one another by rotating around single bonds. They go by the name conformational isomers as well. Conformational isomerism in chemistry is a type of stereoisomerism where the isomers can be changed simply by rotating around formally single bonds Although any two atomic configurations in a molecule that differ by rotation about a single bond can be referred to as different conformations. Conformations that specifically correspond to local minima on the potential energy surface are referred to as conformational isomers or conformers.
The transition states between the local-minimum conformational isomers are conformations that match local maxima on the energy surface. One must pass a rotational energy barrier to interconvert one conformer to another during rotations about single bonds. A Free rotation occurs when the energy barrier is low, and a sample of the compound exists as a quickly equilibrating mixture of various conformers. The restricted rotation occurs when the energy barrier is high enough, and a molecule may exist for a considerable amount of time as a stable rotational isomer or rotamer (an isomer arising from hindered single-bond rotation).
Explanation
Two carbon atoms are joined by a single bond in the compound ethane, and each carbon is also joined to three hydrogen atoms. There are an endless number of conformations that can be formed depending on the angle of rotation if one of the carbon atoms in ethane is allowed to rotate around the central link while the other is maintained constant.
The relative spatial configurations of the hydrogen atoms of one carbon atom in relation to the hydrogen atoms of the other carbon atom vary between each of these conformations. However, the varied bond angles and bond lengths are constant throughout all of these conformations.
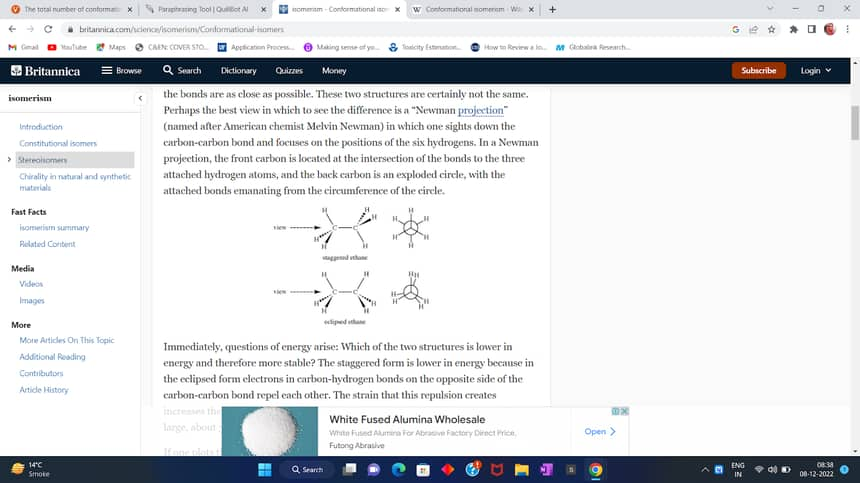
Ethane can exist in two extreme conformations out of an infinite number of potential conformations. These conformations are eclipse and staggered.
The tetrahedrally organised hydrogen atoms of both carbon atoms are facing one another in the eclipsed conformation, and the carbon-hydrogen bond pairs between the two carbon atoms are quite near to one another.
The tetrahedrally organised hydrogen atoms of both carbon atoms are situated far apart in the staggered conformation, as are the bond pairs forming between the two carbon atoms' hydrogen atoms.
Conclusion
The potential energy of the molecule fluctuates slightly when it rotates around the carbon-carbon single bond because rotation around the single bond is not completely free.
The two carbon atoms are widely apart in the staggered form, which has the lowest potential energy.
When the two carbon atoms are in the eclipsed conformation, which is more prevalent, it increases with rotation and reaches its maximum value.
Which of the two structures is more stable and has a lower energy level?
Due to the fact that in the eclipsed form, electrons in carbon-hydrogen bonds on the opposite side of the carbon-carbon bond resist one another, the staggered form has a lower energy. The tension this repulsion causes raises the eclipsed form's potential energy.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
