How Many Bridging Oxygen Atoms are Present in P4O10
Introduction
Phosphorous pentoxide is the common name of the compound with the chemical formula P4O10. Phosphorous pentoxide comprises 4 phosphorous (P) atoms and 10 oxygen (O) atoms. Phosphorous pentoxide is a covalent compound (a molecule formed by covalent bonds). It is a compound in which the atoms share one or more pairs of valence electrons rather than donating them). The number of bridging atoms present in a molecule of phosphorous pentoxide is 6.
Phosphorous pentoxide is sometimes also referred to as diphosphorus pentoxide, phosphoric anhydride, and tetra phosphorus decaoxide.
General properties of phosphorous pentoxide
Phosphorous pentoxide is a solid, white, and waxy substance at room temperature that comes in 4 different crystalline structures. It is an anhydride of phosphoric acid and is too hygroscopic (a substance that has a tendency to absorb moisture from nature). Also, phosphorus pentoxide is often used as a desiccating agent to keep places dry and free from atmospheric moisture.
When phosphoric pentoxide is stored for a long time, it reacts with the atmosphere to form a thin layer of phosphoric acid around the compound. This layer of phosphoric acid prevents phosphorous pentoxide from absorbing water out of the air, which makes it less effective as a desiccant. To prevent this process, phosphorous pentoxide is usually made in granular form for desiccant use.
Physical properties of phosphorous pentoxide
Phosphorous pentoxide is unique in the way it can exist in up to 4 distinct polymorphs.
The most common form of a polymorph of phosphorous pentoxide is a single molecule of P4O10, which is formed by the cohesion of two smaller P2O5 molecules.
As P4O10 has a very unstable molecular configuration, two moles join up to form a larger single molecule of P4O10.
The configuration of the P4O10 is structured as 4 tetrahedra, each sharing a leg with another.
Each tetrahedron of this structure is composed of a central phosphorous atom surrounded by 4 oxygen atoms, where the 3 atoms at the base of the tetrahedron are shared.
A single molecule of phosphorous peroxide looks somewhat like a small hexagonal cell with terminal oxygen atoms protruding out from the sides.
The particular configuration of the molecules makes the phosphorus pentoxide less dense than most other crystalline solids, with a density of only 2.3g/cm3.
The geometric structure of phosphorus pentoxide looks like the hydrocarbon crystal adamantane, and it has a relatively high melting point of 340^{\circ}C (
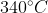 ) for covalently bonded compounds.
) for covalently bonded compounds. The boiling point of phosphorous pentoxide is just 20^{\circ}C (
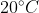 ) more than its melting point, so the compound often skips melting and sublimes directly into a gas.
) more than its melting point, so the compound often skips melting and sublimes directly into a gas.The hexagonal cell of a molecule of phosphorous pentoxide is bonded together by weak van der Waals forces (an electrostatic attraction between molecules).
A phosphorous pentoxide molecule contains 6 P–O–P bonds (each is sp3 hybridised) and 4 P=O bonds.
The dipole-dipole interactions of the P–O–P bond keep the molecule together. P–O–P bonds are polar in nature with a negative valence on the oxygen atom.
All the polymorphs of phosphorous pentoxide are based on the tetrahedral arrangements of the phosphorous and oxygen atoms.
Chemical properties of phosphorous pentoxide
Phosphorous pentoxide is a polar compound in nature.
The electronegativity difference between the oxygen atom and the phosphorus atom is 1.4, thus making P–O bonds rather polar in nature.
Even though phosphorous pentoxide is polar in nature, it will not get dissolved in water because it instead undergoes exothermic hydrolysis.
Phosphorous pentoxide is an anhydride (it is formed by removing water from a compound).
It is the corresponding anhydride of the phosphoric acid (H3PO4) and reacts violently with water (H2O) to form phosphoric acid.
The equation is given below -
P_{4}O_{10}+6H_{2}O\to 4H_{3}PO_{4}
![]()
The enthalpy (total heat content of a system) of the reaction is -177kJ/mol, meaning that for every 1 mole of P4O10, 177kJ of energy is released in the form of heat.
This reaction of phosphorous pentoxide with water is one of the main methods of producing industrial amounts of phosphoric acid, an extremely important ingredient in fertilisers.
Phosphorous pentoxide is non-combustible by nature and does not react with oxygen to produce a flame.
Phosphorous pentoxide however, releases heat (exothermic) when the hydrolysis reaction of phosphorous pentoxide with water and water-containing substances takes place.
This reaction can release enough energy to catalyze a combustion reaction between the water-containing material and the atmosphere.
Phosphorous pentoxide is very corrosive to the metal in nature and forms various metal oxides and phosphate metals when brought into contact with metals.
Phosphorous pentoxide is also very corrosive to human skin and can cause chemical burns and respiratory inflammation, even at low concentrations.
Uses of phosphorous pentoxide
Phosphorous pentoxide is mainly used as a desiccant because it readily reacts with water. Phosphorous pentoxide can draw little traces of water out of the atmosphere to keep its surroundings dry and moisture free. The hydrolysis of water and phosphorous pentoxide creates a gummy layer of phosphoric acid, which can inhibit its water-removing properties.
The desiccant properties of phosphorous pentoxide are frequently used to convert a number of acids into their corresponding anhydrides, like Phosphorous pentoxide converts nitric acid (HNO3) into its anhydride dinitrogen pentoxide (N2O5).
The majority of phosphorous pentoxide is not used for desiccating purposes but is used as an intermediating reactant to create other compounds. Phosphorous pentoxide is used to dehydrate organic compounds like amides, which are nitriles (an important class of organic molecules used in rubber manufacturing and lab procedures).
Notes
The number of sigma bonds present in a molecule of phosphorous pentoxide is 16.
Phosphorous pentoxide is an anhydride of phosphoric acid (H3PO3).
It has a higher s% character in the P–O bond than P4O6.
It has a cage-like structure.
It has four sp3 hybridised phosphorus atoms.
It has p_{\pi }-d_{\pi } (![]() ) bonding.
) bonding.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
