How Many Bravais Lattices are There
Introduction
An infinite collection of discrete points known as a Bravais lattice is produced by a series of discrete translation operations specified in three dimensions. The term comes from Auguste Bravais (1850).
R=n_{1}a_{1}\pm n_{2}a_{2}\pm n_{3}a_{3}![]()
where the n_{i}![]() are arbitrary values, are primitive translation vectors that span lattices or primitive vectors with a wide range of potential directions (though not necessarily parallel ones). There are various fundamental vector alternatives for a specific Bravais lattice. No matter which direction is chosen, a fundamental property of all Bravais lattices is that they all appear the same from each discrete lattice point.
are arbitrary values, are primitive translation vectors that span lattices or primitive vectors with a wide range of potential directions (though not necessarily parallel ones). There are various fundamental vector alternatives for a specific Bravais lattice. No matter which direction is chosen, a fundamental property of all Bravais lattices is that they all appear the same from each discrete lattice point.
The (restricted) boundaries of a crystalline structure are precisely defined by the Bravais lattice concept. Each crystal's lattice point has one or more atoms that are present and are frequently referred to as the basis or theme. The basis, which is located inside the lattice, can be composed of solid matter atoms, molecules, or polymer threads.
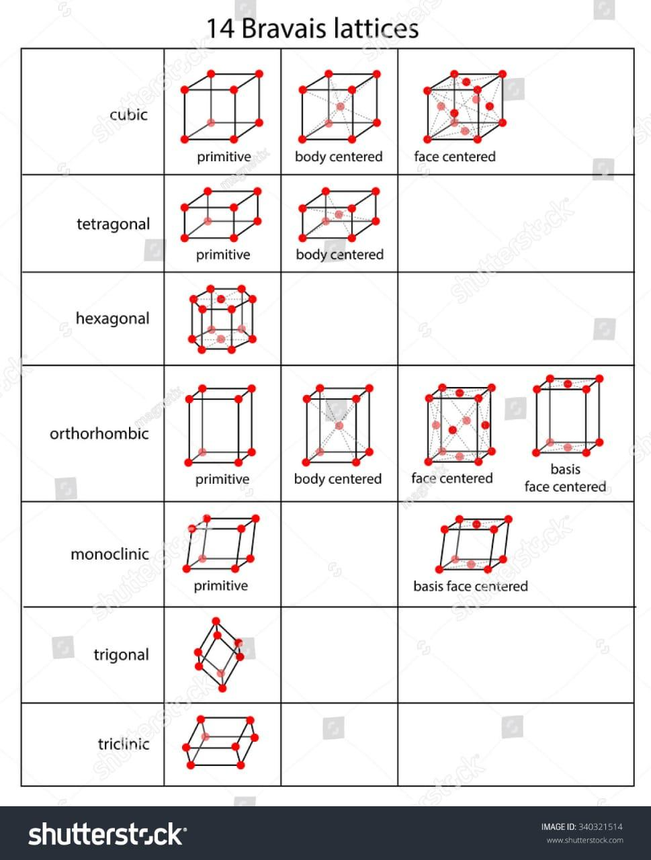
It is frequently compared when two Bravais lattices have the same symmetry groups. There are therefore only 5 possible Bravais lattices in a two-dimensional space, but 14 in a three-dimensional setting.
There is a chance that Bravais lattice symmetry groups will exist for 14 of the 230 space groups. The space group refers to these Bravais lattices as Bravais classes, Bravais flocks, Bravais arithmetic classes, or Bravais lattices classification.
14 Bravais Lattices
1. Simple Cubic
2. Face-Centred Cubic
3. Body-Centred Cubic
4. Hexagonal
5. Rhombohedral
6. Simple Tetragonal
7. Body-Centred Tetragonal
8. Simple Orthorhombic
9. Face-Centred Orthorhombic
10. body-Centred Orthorhombic
11. Base-Centred Orthorhombic
12. Simple Monoclinic
13. Base-Centred Monoclinic
14. Triclinic
Examples
These are the elements: hydrogen (g), helium (g), beryllium (s), magnesium (s), cobalt (s), zinc (s), yttrium (s), zirconium (s), ruthenium (s), cadmium (s), tellurium (s), gadolinium (s), terbium (s), dysprosium (s), holmium (s), erbium (s), and thulium (s).
Most hexagonal elements are packed (solid), with the exception of Carbon (s), Nitrogen (g), Lanthanum (s), Praseodymium (s), Neodymium (s), Promethium (synthetically produced), Curium (synthetically produced), Berkelium (synthetically produced), Californium (synthetically produced), Einsteinium (synthetically produced), Americium (synthetically produced), and Selenium (Se).
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
