How Many ATP Does the Electron Transport Chain Produce?
32 molecules of ATP are generated in the electron transport chain.
Four protein complexes make up the electron transport chain (ETC), also referred to as the oxidative phosphorylation process, which combines redox events to produce an electrochemical gradient that results in the synthesis of ATP. The mitochondria are the sites of both cellular respiration and photosynthesis. In the first, the organic molecules break down, releasing both energy and electrons. In the latter, after being activated by light, the electrons join the chain, and the energy that is released is used to produce carbohydrates.
An organic substance called adenosine triphosphate (ATP) provides energy for a number of biological processes in living cells, such as muscle contraction, nerve impulse transmission, condensate dissolution, and chemical synthesis. Because it is found in all known forms of life, ATP is frequently referred to as the "molecular unit of currency" of intracellular energy transfer. Either adenosine monophosphate (AMP) or adenosine diphosphate (ADP) are produced during metabolic processes.
Yield:
A FADH2 molecule produces only two molecules of ATP in comparison to three NADH molecules.
In the ETS, ten NADH molecules and two FADH2 molecules are produced.
These NADH and FADH2 molecules consequently generated a total of 34 molecules of ATP.
In eukaryotic organisms, the inner mitochondrial membrane houses both the electron transport chain and the site of oxidative phosphorylation. The electron transport chains are found in the plasma membrane of a prokaryotic, unicellular organism because mitochondria are absent in these organisms.
Electron Transport Chain
Two molecules of pyruvate, each with three carbon atoms, are formed from a glucose molecule with six. The name of this procedure is glycolysis. If the current bacterium has an aerobic metabolism, the pyruvate molecule can instead be converted to acetyl-Coenzyme A (acetyl-CoA) in an aerobic environment. The acetyl group in acetyl-CoA contains two carbon atoms as a result of the separation of carbon dioxide (CO2), which allows it to now enter the Krebs cycle (also known as the citric acid cycle).
In eukaryotes, the electron transport chain pumps protons into the intermembrane gap to create the electrochemical gradient over the inner mitochondrial membrane using energy from interactions between oxygen and reduced substances like cytochrome c and (indirectly) NADH and FADH2. The electron transport chain is located on the thylakoid membrane in eukaryotes that are capable of photosynthesis. ATP is created as a result of the proton gradient created by the electron transport of the proton pump.
The four pathways that comprise the entire cellular process are the Krebs cycle, oxidative phosphorylation, glycolysis, and pyruvate oxidation.
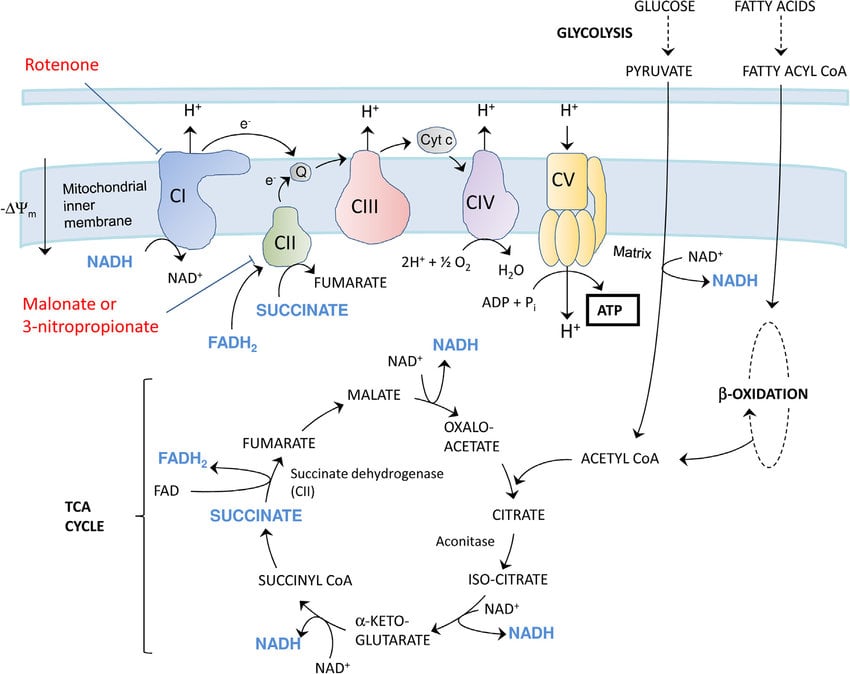
Pyruvate oxidation: Pyruvate is transformed into an acetyl group attached to a coenzyme. A carrier molecule in the presence of oxygen. However, the citric acid cycle is typically where the acetyl group is delivered for further catabolism. The resulting acetyl CoA can enter a number of pathways.
Krebs cycle: The Krebs cycle, also referred to as the TCA cycle (tricarboxylic acid cycle), is a set of chemical reactions that releases energy from stored sources by oxidising acetyl-CoA, which is derived from proteins, fats, and carbohydrates. It is also known as the CAC (citric acid cycle). Instead of fermenting, organisms that respire use the Krebs cycle to generate energy, either through anaerobic or aerobic respiration. The cycle also provides the reducing agent NADH and some amino acid precursors that are required in a number of other reactions.
Oxidative Phosphorylation: The process of oxidative phosphorylation uses the energy produced by these oxidation and reduction processes to drive the synthesis of ATP from ADP. This process involves coupling electrons from NADH and FADH2 with O2.
Glycolysis: Glycolysis is the metabolic process by which glucose is broken down to produce energy. It also produces ATP, NADH, water, and two pyruvate molecules. The entire process, which takes place in the cytoplasm of a cell, doesn't require oxygen.
Adenosine triphosphate (ATP)
Chemical Formula: \\C_{10}H_{16}N_{5}O_{13}P_{3}
![]()
Structure :
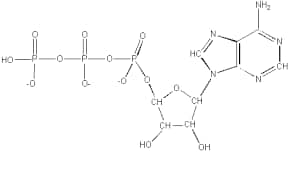
Adenosine triphosphate (ATP) is the form of energy used and stored at the cellular level. The structure of ATP, a nucleoside triphosphate, consists of adenine, ribose, and three serially bound phosphate groups. It is sometimes referred to as the "energy currency" of the cell because ATP connects the second and third phosphate groups, where it stores easily usable energy. Along with providing energy, ATP hydrolysis supports a variety of cellular processes, such as DNA and RNA synthesis and signalling. Several catabolic processes, including cellular respiration, beta-oxidation, and ketosis, supply the energy needed to produce ATP.
Examples
Examples of electron transport chains include the chemosynthetic process in prokaryotic bacteria and the mitochondrial energy production in eukaryotes.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
