How Many Atoms are in One Mole of a Substance
Introduction
12 grams of pure carbon-12 weigh exactly one mole, or \mathrm{6.022\times 10^{23}} ![]() atoms, making one mole equivalent to 12 grams of pure carbon-12. Avogadro's Number is the number of particles included in one mole. Which is \mathrm{6.022\times 10^{23}}
atoms, making one mole equivalent to 12 grams of pure carbon-12. Avogadro's Number is the number of particles included in one mole. Which is \mathrm{6.022\times 10^{23}} ![]()
Mole
Because of this, each atom in each element has a different mass. For instance, a typical iron atom will have a mass of 55.8 amu. Remember that the mass of a proton or neutron, 1 amu, is quite little. Since chemical lab balances do not measure amu, we will need to scale this up to something they do: grammes. How many amu are required to create one gramme? There is a huge, huge answer to \mathrm{6.022\times 10^{23}} ![]()
That amounts to 602, 000, 000, 000,000. Even the name of that number escapes me. That's alright because this number has a unique name (kind of like 12 has its special name: dozen). It has a unique name called Avogadro's number, after this person. The term "mole" is a more popular nickname for Avogadro's number. A mole is simply a big number that you may use to count how many of something you have. It is also a big number. It consistently equals \mathrm{6.022\times 10^{23}} ![]() .
.
Applications of mole
The molar mass of a substance is the weight of a mole of that substance. In chemistry, the molar mass is frequently employed to convert grammes of a chemical to moles. The periodic table lists an element's molar mass, which is its atomic weight in grammes per mole (g/mol). The number of moles in a substance can be estimated if its mass is known. One mole of substance/molar mass of the substance is the conversion factor needed to go from a substance's mass in grammes to moles.
The chemical compound composition can also be understood in terms of the mole notion. Take methane, and CH4, for instance. According to this molecule and its molecular formula, methane has 1 mole of carbon and 4 moles of hydrogen per mole. The mole is used here as a standard unit that may be used to calculate a ratio as indicated below:
\mathrm{2\ mol(H)+1\ mol(O)= H{_{2}}O}
![]()
The moles of H and O in this chemical reaction refers to the number of atoms of each element that combine to generate 1 mol of H2O.
One should compare a mole to quantities like a dozen or a pair to understand what it signifies. A mole is defined as 6.022141791023 of anything, much as a pair can be defined as two shoes, two books, two pencils, two persons, or two of anything else. Using the relationship shown below:
\mathrm{1\ mol=6.022\times 10^{23}}
![]()
Because of how big Avogadro's constant is, it is very challenging to picture a mole of something. Think about the size of a single grain of wheat, for instance. The total number of wheat grains tallied would be far less than Avogadro's constant even if every person who has ever lived did nothing but count individual grains of wheat for the rest of their life; the quantity of wheat produced throughout history does not even come close to Avogadro's Number.
Counting Atoms
Measure out 56 trillion iron atoms at the balance. After that, add 108 billion oxygen molecules to them. How are 56 trillion iron atoms intended to be measured out?
Atoms must be counted and measured in some way, but due to their small size and the fact that we typically deal with large numbers of them, a different approach must be taken.
Counting out 600 oranges might take some time. Alternatively, you could keep putting oranges on a scale until you hit 250 pounds (50 dozen times 5 pounds each dozen equals 250 pounds). I would choose the second option since it would eliminate the need for me to keep track of oranges and worry about losing count. All I'd have to do is concentrate on collecting 250 pounds of oranges.
Chemistry uses a similar concept because counting individual atoms would be absurd. Once more, like with the oranges, we will use their weight as a counting aid. Will we then weigh them in pounds after sorting them into dozens? Most likely not. We will apply what we already understand about an atom's mass or weight.
On the periodic table, the atomic weight can be seen directly beneath the symbol. What changes as the atomic number (the highest number in each box) rises? On the periodic table, you should observe that the atomic number (the number of protons) increases by 1 and the atomic weight also increases as you move from left to right and top to bottom. Each element's atoms become heavier as a result of holding more protons and neutrons (and, to be fair, electrons as well, though they don't affect the mass).
How Was The Avagadro Number Calculated?
Avagadro was a child during a crucial time in the history of chemistry. Chemists like John Dalton and Joseph Louis Gay-Lussac discussed how these infinitesimally small particles behaved as they started to comprehend the fundamental characteristics of atoms and molecules. Avagadro was particularly intrigued by Gay-law Lussac's mixing volumes.
Avogadro played about with the implications of this law and predicted that for it to hold, equivalent volumes of any two gases at comparable weights and temperatures must hold an equivalent number of particles.
And the only way to prove that this law might be accurate is if there were a distinction between atoms and molecules and if certain elements, like nitrogen, genuinely existed as molecules ( N2 rather than simply N). Given that Avogadro lacked terminology like "molecule" to describe his hypothesis and that his ideas encountered resistance from people like John Dalton, among others.
Another chemist, Stanislao Cannizzaro, was required to give Avogadro's theories the attention they deserved. Avogadro had already passed away by the time their concepts acquired popularity.
Avogadro’s Number
Avogadro's number or Avogadro's constant refers to the number of units contained in one mole of any substance. The value is 6.0221408571023. Depending on the nature of the reaction and the substance, the units may be electrons, ions, atoms, or molecules.
Key Points
The value of Avogadro’s Number is 6.0221408571023.
1 mol of a substance contains \mathrm{6.022\times 10^{23}}
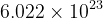 .
.A substance's mass is measured at the atomic level using an atomic mass unit. The weight of one carbon atom divided by twelve is the definition of an atomic mass unit.
The amount of C-12 atoms in 12 g of carbon 12 is one mol.
Chemists like John Dalton and Joseph Louis Gay-Lussac discussed how these infinitesimally small particles behaved as they started to comprehend the fundamental characteristics of atoms and molecules
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
