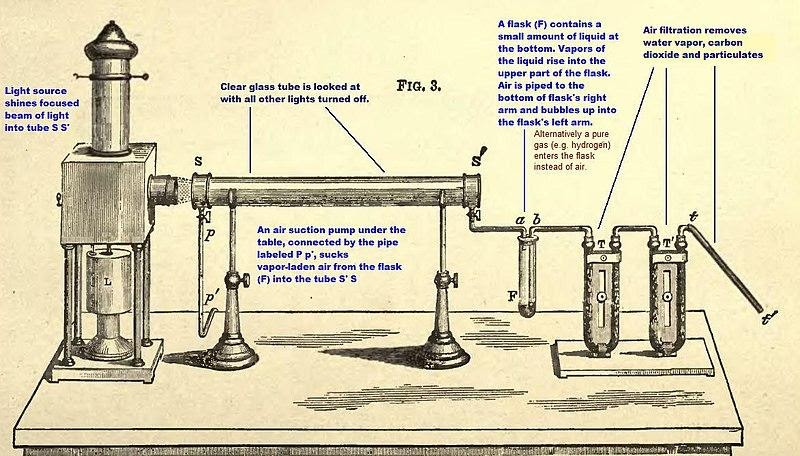
1. What are the circumstances of Tyndall's outcome?
Two conditions you should be satisfied to see the effect of Tyndall: The diameter of the dispersed particles should be less than the length of light used. The return indicators for the distribution rate and the dispersed phase should differ by the size of the maximum scale.
2. What solution is needed for Tyndall's outcome?
The Tyndall effect is due to scattering of light in the colloidal scattering, while the lack of light is a real solution. This result is used to determine whether the mixture is a real solution or a colloid.
3. Why does the Tyndall effect occur?
It is caused by the light of the incident radiation from the particles, from the inner walls of the particles, and the retraction and separation of the radiation as it passes through the particles. Some eponyms include Tyndall beam (light dispersed by colloidal particles).
4. How can we see the effect of Tyndall?
Distribute light through water droplets in the air. Lighting a flashlight in a milk glass. One of the most intriguing examples of Tyndall effect is the iris in blue. The flexible layer over the metal that causes the spread of blue light makes the eyes look blue.
5. What is the effect of Tyndall effect dispersion of light l and its use?
The effect of Tyndall is the effect of light scattering on colloidal scattering while not showing scattering in real solution. This result is used to determine whether the mixture is a real solution or a colloid.
6. Where is the Tyndall effect dispersion of light l effect used?
The Tyndall effect is used in commercial and laboratory settings to determine the particle size of aerosols. Opalescent glass shows the effect of Tyndall. The glass looks blue, but the bright light on it looks orange. The colour of the blue eyes comes from Tyndall, which spreads through the flexible layer above the eyeball.
7. Does the water show Tyndall's effect?
Water and milk form a colloidal solution that reflects the effect of Tyndall because the larger particles that cause light scattering and reflect the effect of Tyndall.
8. Does sugar indicate a Tyndall effect dispersion of light l effect?
Since colloids have particles in them that transmit the transmitted light, they reflect Tyndall's influence. The sugar solution is a real solution and not a colloid solution. Therefore, the effect of Tyndall is not indicated by the sugar solution.
9. Does the soap solution reflect the effect of Tyndall effect dispersion of light l?
The aqueous solution below the critical micelle concentration is not a colloid solution and the soapy water solution above the critical micelle concentration is a colloidal solution. Therefore, the effect of Tyndall effect dispersion of light l will be reflected in the gift solution over the delicate micelle concentration.
10. Who discovered Tyndall effect?
Tyndall's effect was first discovered (and named) by Irish naturalist John Tyndall.
11. What do you mean by Tyndall effect?
The effect of Tyndall is that the particles on the particle emit rays of light directed toward them. This effect of Tyndall effect dispersion of light is demonstrated by all colloidal solutions and other excellent suspensions.
12. What is the Tyndall effect?
The Tyndall effect is the scattering of light by colloidal particles in a solution. When a beam of light passes through a colloidal solution, the particles scatter the light, making the path of the light beam visible.
13. Why can't we observe the Tyndall effect in true solutions?
True solutions contain dissolved particles that are too small to scatter light effectively. The particles in true solutions are typically smaller than the wavelength of visible light, so they don't cause noticeable scattering.
14. Can the Tyndall effect be used to distinguish between a solution and a colloid?
Yes, the Tyndall effect can be used to distinguish between a solution and a colloid. When a light beam is passed through a colloid, the path of the light becomes visible due to scattering, while in a true solution, the light beam remains invisible.
15. How does particle size affect the Tyndall effect?
Particle size greatly influences the Tyndall effect. Larger particles (but still small enough to remain suspended) scatter light more effectively, producing a more pronounced Tyndall effect. As particle size decreases, the scattering becomes less noticeable.
16. What is the relationship between wavelength of light and the Tyndall effect?
The Tyndall effect is more pronounced for shorter wavelengths of light. Blue light, having a shorter wavelength, is scattered more than red light, which has a longer wavelength. This is why scattered light often appears bluish.
17. How does the Tyndall effect differ from Rayleigh scattering?
The Tyndall effect occurs when light is scattered by larger colloidal particles, while Rayleigh scattering happens when light is scattered by very small particles or molecules. The Tyndall effect is more pronounced and visible to the naked eye.
18. How does the Tyndall effect relate to colloid stability?
The Tyndall effect is an indicator of colloid stability. A strong Tyndall effect suggests that the colloidal particles are well-dispersed and stable in the medium. If the Tyndall effect weakens over time, it may indicate that the colloid is becoming unstable or coagulating.
19. How does the Tyndall effect relate to Brownian motion?
While the Tyndall effect and Brownian motion are distinct phenomena, they are both characteristics of colloidal systems. The Tyndall effect makes the presence of colloidal particles visible through light scattering, while Brownian motion describes the random movement of these particles. The combination of these effects contributes to the overall behavior and stability of colloids.
20. What is the difference between dynamic and static light scattering in relation to the Tyndall effect?
Dynamic light scattering measures the fluctuations in scattered light intensity over time, providing information about particle size and movement. Static light scattering, which is more closely related to the Tyndall effect, measures the average intensity of scattered light to determine particle size and shape.
21. Can the Tyndall effect be observed in all types of colloids?
The Tyndall effect can be observed in most types of colloids, including sols (solid particles in liquid), emulsions (liquid in liquid), and aerosols (liquid or solid in gas). However, the intensity of the effect may vary depending on the nature and concentration of the dispersed particles.
22. What causes the blue color of the sky?
The blue color of the sky is a result of Rayleigh scattering, which is similar to the Tyndall effect. Air molecules scatter shorter wavelengths (blue light) more than longer wavelengths, giving the sky its blue appearance.
23. What is the role of polarization in the Tyndall effect?
Polarization plays a significant role in the Tyndall effect. The scattered light is often partially polarized, with the degree of polarization depending on the scattering angle and particle properties. This polarization can be used to enhance the visibility of the Tyndall effect or to gather additional information about the colloidal system.
24. Can the Tyndall effect be used to estimate particle size in colloids?
While the Tyndall effect alone cannot provide precise particle size measurements, it can give a rough estimate. The intensity and color of scattered light can provide qualitative information about particle size. For more accurate measurements, techniques like dynamic light scattering are used in conjunction with Tyndall effect observations.
25. How does the shape of colloidal particles influence the Tyndall effect?
The shape of colloidal particles can significantly influence the Tyndall effect. Non-spherical particles, such as rods or platelets, can cause anisotropic scattering, leading to variations in the intensity and polarization of scattered light depending on the orientation of the particles.
26. Can the Tyndall effect be used to study the kinetics of colloidal aggregation?
Yes, the Tyndall effect can be used to study colloidal aggregation kinetics. As particles aggregate, the intensity and nature of scattered light change, allowing researchers to monitor the progress of aggregation in real-time. This can provide insights into the stability and behavior of colloidal systems.
27. How does temperature affect the Tyndall effect?
Temperature can indirectly affect the Tyndall effect by influencing the properties of the colloidal system. Higher temperatures may increase Brownian motion, potentially enhancing the Tyndall effect. Temperature changes can also affect the solubility and stability of colloidal particles, which in turn impacts light scattering.
28. Can the Tyndall effect be used in nanotechnology applications?
Yes, the Tyndall effect has applications in nanotechnology. It can be used to detect and characterize nanoparticles in solution, monitor the formation of nanostructures, and assess the stability of nanomaterials. The sensitivity of light scattering to particle size makes it valuable in nanoscale research and development.
29. What is the significance of the Tyndall effect in photonics and optical fiber technology?
In photonics and optical fiber technology, the Tyndall effect can be both beneficial and detrimental. While it can be used to visualize the path of light in optical fibers for diagnostic purposes, unwanted scattering (similar to the Tyndall effect) can lead to signal loss and degradation in optical communication systems.
30. How can the Tyndall effect be used in environmental monitoring?
The Tyndall effect is valuable in environmental monitoring for detecting and measuring particulate matter in air and water. It can be used to assess air quality by detecting aerosols and dust particles, and to monitor water quality by identifying suspended solids and colloidal contaminants in water bodies.
31. Can the Tyndall effect occur in gases?
Yes, the Tyndall effect can occur in gases if they contain suspended particles or aerosols. For example, the visibility of a light beam in a smoky room is due to the Tyndall effect.
32. What role does the Tyndall effect play in atmospheric science?
In atmospheric science, the Tyndall effect helps explain various phenomena such as the visibility of light beams in foggy or dusty air, the appearance of crepuscular rays (sunbeams), and the scattering of light by aerosols and particulates in the atmosphere.
33. How can the Tyndall effect be used in water quality testing?
The Tyndall effect can be used to detect the presence of colloidal particles in water. By shining a light through a water sample, the visibility of the light beam can indicate the presence of suspended particles, helping to assess water clarity and purity.
34. How does the Tyndall effect contribute to the appearance of natural phenomena like rainbows?
While rainbows are primarily caused by refraction and reflection of light in water droplets, the Tyndall effect can enhance their visibility. The scattering of light by water droplets and other particles in the air makes the path of light more visible, contributing to the vivid appearance of rainbows.
35. How does the concentration of colloidal particles affect the Tyndall effect?
Generally, a higher concentration of colloidal particles leads to a more pronounced Tyndall effect. As the number of particles increases, there are more opportunities for light scattering, making the path of the light beam more visible. However, extremely high concentrations may lead to multiple scattering events, potentially reducing the clarity of the effect.
36. How does the refractive index difference between the dispersed phase and the dispersion medium affect the Tyndall effect?
The greater the difference in refractive index between the dispersed particles and the dispersion medium, the more pronounced the Tyndall effect. A larger refractive index difference leads to stronger light scattering, making the path of the light beam more visible.
37. What is the significance of the Tyndall effect in biological systems?
In biological systems, the Tyndall effect can be observed in various contexts, such as the scattering of light by protein aggregates or cellular organelles. It plays a role in the visual appearance of biological fluids and can be used as a diagnostic tool in some medical applications, like detecting protein aggregation in eye diseases.
38. What is the relationship between the Tyndall effect and turbidity?
The Tyndall effect and turbidity are closely related. Turbidity is a measure of the cloudiness or haziness of a fluid caused by suspended particles, which is essentially what the Tyndall effect makes visible. A stronger Tyndall effect generally indicates higher turbidity in a colloidal system.
39. How does the Tyndall effect differ in forward and backward scattering?
In forward scattering (light scattered in the same direction as the incident beam), the Tyndall effect is generally stronger and may appear more bluish. In backward scattering (light scattered opposite to the incident beam), the effect is usually weaker and may appear more reddish. This difference is due to the wavelength dependence of light scattering.
40. How does the Tyndall effect relate to the concept of optical transparency?
The Tyndall effect is inversely related to optical transparency. Materials that exhibit a strong Tyndall effect appear less transparent because light is scattered rather than transmitted directly. True solutions, which don't show the Tyndall effect, are generally more optically transparent than colloidal dispersions.
41. What is the role of the Tyndall effect in understanding the properties of aerosols?
The Tyndall effect is crucial in studying aerosols, which are colloidal systems of solid or liquid particles suspended in a gas. It helps in visualizing the presence and distribution of aerosol particles, understanding their light-scattering properties, and assessing their concentration and behavior in the atmosphere.
42. How can the Tyndall effect be used to study the stability of emulsions?
The Tyndall effect can be used to monitor the stability of emulsions over time. As an emulsion destabilizes and particles begin to coalesce or separate, changes in the intensity and nature of scattered light can be observed. This provides a non-invasive way to assess emulsion stability and quality.
43. How does the Tyndall effect contribute to the appearance of opalescent materials?
The Tyndall effect plays a key role in the appearance of opalescent materials. These materials contain microscopic particles that scatter light, creating a milky, iridescent appearance. The size and distribution of these particles determine the colors and intensity of the scattered light, resulting in the characteristic opalescent look.
44. Can the Tyndall effect be used to detect impurities in pharmaceuticals?
Yes, the Tyndall effect can be used as a quality control measure in pharmaceutical production. By observing light scattering in liquid medications or solutions, it's possible to detect the presence of unwanted particles or impurities that might not be visible to the naked eye, ensuring product purity and safety.
45. How does the Tyndall effect relate to the concept of critical micelle concentration (CMC) in surfactant solutions?
The Tyndall effect can be used to detect the formation of micelles at the critical micelle concentration (CMC) in surfactant solutions. As micelles form, they create a colloidal dispersion that scatters light, resulting in a visible Tyndall effect. This change in light scattering behavior can be used to determine the CMC of surfactant systems.
46. What is the relationship between the Tyndall effect and Mie scattering?
The Tyndall effect is a manifestation of Mie scattering when the scattering particles are of a size comparable to or larger than the wavelength of light. Mie scattering theory provides a more comprehensive mathematical description of light scattering by spherical particles, encompassing both the Tyndall effect and Rayleigh scattering.
47. What role does the Tyndall effect play in the appearance of certain gemstones?
Some gemstones, like opals and moonstones, owe their unique appearance partly to the Tyndall effect. These stones contain microscopic structures that scatter light, creating their characteristic play of colors or adularescence. The size and arrangement of these structures determine the specific optical effects observed.
48. How does the Tyndall effect influence the design of light-scattering based sensors?
The Tyndall effect is fundamental to the design of many light-scattering based sensors. These sensors utilize the principles of light scattering to detect and measure particles in various media. The intensity and angular distribution of scattered light can provide information about particle size, concentration, and other properties, making such sensors valuable in numerous applications.
49. Can the Tyndall effect be used to study the formation of gels?
Yes, the Tyndall effect can be used to study gel formation. As a solution transitions into a gel, the formation of a network structure often results in increased light scattering. By monitoring changes in the Tyndall effect over time, researchers can gain insights into the kinetics and mechanisms of gel formation in various systems.
50. How does the Tyndall effect relate to the concept of colloidal gold?
Colloidal gold solutions exhibit the Tyndall effect due to the presence of suspended gold nanoparticles. The color of colloidal gold, which can range from red to blue depending on particle size, is a result of both absorption and scattering of light. The Tyndall effect helps in visualizing and characterizing these colloidal gold solutions.
51. What is the significance of the Tyndall effect in understanding atmospheric optics?
In atmospheric optics, the Tyndall effect helps explain various phenomena such as the blue color of the sky, the reddish appearance of sunsets, and the visibility of light beams in foggy or dusty conditions. It provides insights into how light interacts with particles in the atmosphere, influencing our perception of atmospheric optical effects.
52. How can the Tyndall effect be used to study protein aggregation?
The Tyndall effect is useful in studying protein aggregation, a critical issue in biochemistry and pharmaceutical sciences. As proteins aggregate, they form larger particles that scatter light more effectively. By monitoring changes in light scattering intensity, researchers can track the progress of protein aggregation and study factors that influence this process.
53. What is the relationship between the Tyndall effect and dynamic light scattering (DLS) techniques?
While the Tyndall effect demonstrates the presence of colloidal particles through visible light scattering, dynamic light scattering (DLS) techniques analyze the fluctuations in scattered light intensity to determine particle size and distribution. DLS builds upon the principles of the Tyndall effect to provide more detailed quantitative information about colloidal systems.
54. How does the Tyndall effect contribute to the appearance of certain types of clouds?
The Tyndall effect plays a role in the appearance of certain clouds, particularly thin, high-altitude clouds like cirrus. The scattering of sunlight by ice crystals in these clouds can create visible beams or streaks of light, enhancing their visual appearance and contributing to phenomena like sun pillars or light pillars.
55. Can the Tyndall effect be used to detect the presence of microplastics in water?
Yes, the Tyndall effect can be utilized in detecting microplastics in water. By passing a focused beam of light through a water sample, the scattering of light by suspended microplastic particles can be observed. This method, often combined with other analytical techniques, helps in the initial screening and detection of microplastic contamination in aquatic environments.
56. How does the Tyndall effect relate to the concept of light pollution in astronomy?
The Tyndall effect contributes to light pollution in astronomy by scattering artificial light in the atmosphere. When light from cities or other sources is scattered by particles in the air, it creates a diffuse glow in the sky, reducing the visibility of stars and other celestial objects. Understanding this effect is crucial in developing strategies to mitigate light pollution for astronomical observations.
57. What is the role of the Tyndall effect in the development of smart windows?