1. How do you turn Celsius into Kelvin's formula?
Conversion of Celsius into Kelvin:
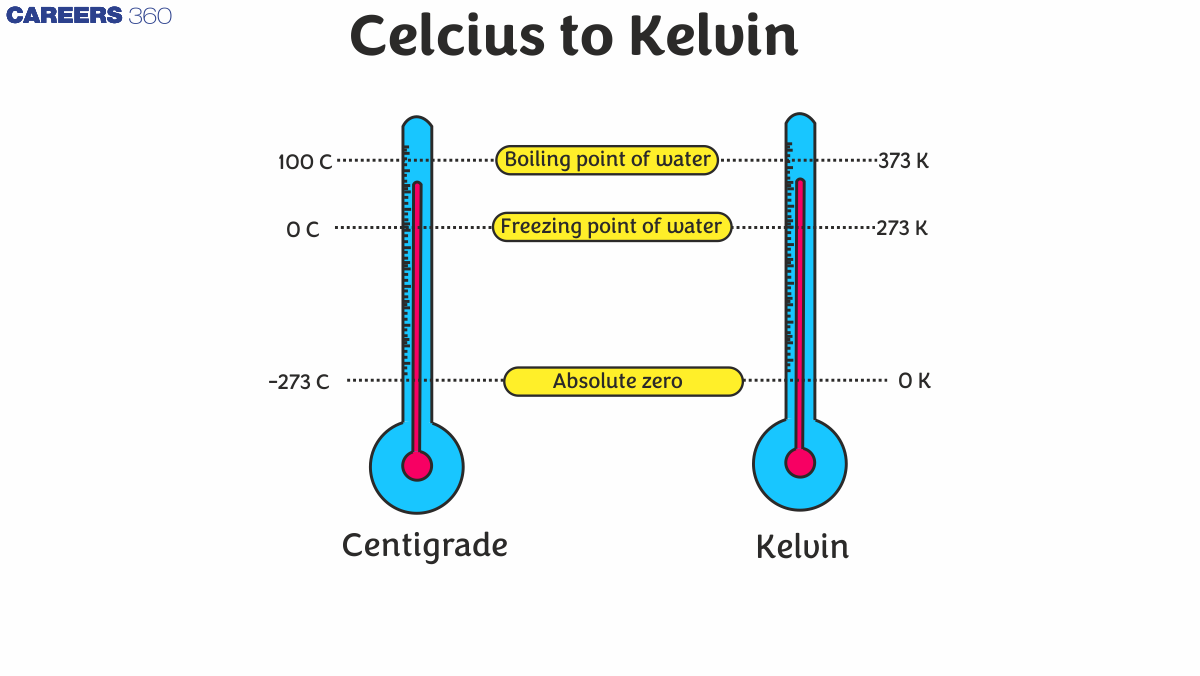
Kelvin = Celsius + 273.15.
2. Is Kelvin Negative possible?
No, it is not possible to have a negative temperature on the Kelvin scale, also known as the absolute temperature scale. This is because absolute zero, which is 0 kelvin, is the lowest possible temperature.
3. What is absolute zero?
Absolute zero is the lowest theoretical temperature, 0 K, or -273.15°C, where all molecular motion stops.
4. Why is 273.15 added in the conversion formula?
The value 273.15 is the difference between the Celsius scale's zero point (water's freezing point) and the Kelvin scale's zero point (absolute zero).
5. Why is the Kelvin scale preferred in science?
The Kelvin scale is preferred because it starts at absolute zero, providing a true representation of thermal energy. It is essential for thermodynamic equations where absolute temperature values are required
6. Why doesn't the Kelvin scale use negative numbers?
The Kelvin scale doesn't use negative numbers because it starts at absolute zero, the lowest possible temperature where particles have no thermal energy. There's no physical meaning to a temperature below this point.
7. Can Kelvin temperatures be negative?
No, Kelvin temperatures cannot be negative. The Kelvin scale starts at absolute zero (0K), which is the lowest possible temperature where particles have no thermal energy.
8. How does the Kelvin scale relate to the concept of absolute zero?
The Kelvin scale is defined with 0K as absolute zero, the theoretical temperature at which all thermal motion of particles stops. This makes it an absolute temperature scale.
9. How does the concept of average kinetic energy relate to the Kelvin scale?
The Kelvin temperature is directly proportional to the average kinetic energy of particles in a substance. At 0K, particles theoretically have no kinetic energy.
10. Why is the Kelvin scale preferred in scientific calculations?
The Kelvin scale is preferred because it's an absolute scale starting at zero thermal energy. This makes calculations involving temperature-dependent properties more straightforward and avoids issues with negative temperatures.
11. What is the formula to convert Celsius to Kelvin?
The formula to convert Celsius to Kelvin is: K = °C + 273.15. This means you simply add 273.15 to the Celsius temperature to get the Kelvin temperature.
12. What is absolute zero in Kelvin and Celsius?
Absolute zero is 0K on the Kelvin scale. This corresponds to -273.15°C on the Celsius scale.
13. What is the Kelvin scale and why is it important in chemistry?
The Kelvin scale is an absolute temperature scale used in scientific measurements. It's important in chemistry because it starts at absolute zero (the lowest possible temperature) and has no negative values, making it ideal for calculations involving temperature-dependent properties of matter and energy.
14. How does the Celsius scale relate to the Kelvin scale?
The Celsius and Kelvin scales have the same size units, but they're offset by 273.15. This means that 0°C is equal to 273.15K, and each 1°C increase corresponds to a 1K increase.
15. Why do we add 273.15 when converting Celsius to Kelvin?
We add 273.15 because that's the difference between the two scales' zero points. 0°C (the freezing point of water) is 273.15K, so adding this value aligns the scales.
16. What's the difference between Kelvin and degrees Kelvin?
There's no difference in value, but "degrees Kelvin" is an outdated term. The correct terminology is simply "Kelvin" without the degree symbol. For example, we say "300 Kelvin" or "300K", not "300 degrees Kelvin".
17. Why is room temperature often given as 298K in chemistry problems?
298K (about 25°C) is commonly used as a standard "room temperature" in chemistry because it's a convenient round number close to typical indoor temperatures and is often used as a reference point in thermodynamic tables.
18. How does the Celsius to Kelvin conversion affect gas law calculations?
Gas law calculations often require temperatures in Kelvin because the volume and pressure of a gas are directly proportional to its absolute temperature. Using Kelvin ensures the calculations work correctly even at very low temperatures.
19. How does the Celsius to Kelvin conversion affect equilibrium constant calculations?
Equilibrium constants often depend on temperature. Using Kelvin ensures that the temperature is always positive and proportional to particle energy, which is crucial for accurate calculations, especially when using equations like the van 't Hoff equation.
20. How does the Celsius to Kelvin conversion affect calculations involving the ideal gas law?
The ideal gas law (PV = nRT) requires temperature in Kelvin. Using Celsius would lead to incorrect results because the equation assumes an absolute temperature scale where 0 represents no thermal energy.
21. How does the Celsius to Kelvin conversion affect calculations involving entropy?
Entropy calculations often involve logarithms of temperature. Using Kelvin ensures these calculations are always valid, as you can't take the logarithm of a negative number (which could happen with Celsius at low temperatures).
22. How does the Kelvin scale relate to the concept of phase transitions?
Phase transitions occur at specific temperatures on both the Celsius and Kelvin scales. However, using Kelvin in calculations ensures that the relationship between temperature and particle energy is always direct and positive, simplifying many thermodynamic equations.
23. How does the Celsius to Kelvin conversion affect calculations involving the Boltzmann distribution?
The Boltzmann distribution, which describes the distribution of particle energies in a system, requires absolute temperature (Kelvin). Using Kelvin ensures the distribution is always mathematically valid and correctly represents the energy states of particles.
24. What's the relationship between the Celsius to Kelvin conversion and the concept of temperature-dependent equilibrium constants?
Equilibrium constants often change with temperature according to the van 't Hoff equation. This equation requires absolute temperature (Kelvin) to work correctly, as it involves logarithms and exponentials that wouldn't make sense with negative temperatures.
25. What's the significance of the temperature 310.15K in biochemistry?
310.15K (37°C) is significant in biochemistry because it's the average human body temperature. Many biochemical reactions and processes are studied at this temperature to mimic physiological conditions.
26. How does the Kelvin scale relate to the concept of critical temperature in phase diagrams?
Critical temperature, the temperature above which a substance cannot exist as a liquid regardless of pressure, is typically expressed in Kelvin. This ensures consistency with other thermodynamic parameters and simplifies calculations in phase equilibria.
27. How does the Celsius to Kelvin conversion affect calculations involving the van der Waals equation?
The van der Waals equation, which describes real gas behavior, requires temperature in Kelvin. Using Kelvin ensures the equation is valid at all temperatures and correctly represents the behavior of gases even at low temperatures.
28. How does the Kelvin scale relate to the concept of activation energy in chemical reactions?
Activation energy, often calculated using the Arrhenius equation, requires temperature in Kelvin. Using Kelvin ensures the equation is valid at all temperatures and correctly represents the temperature dependence of reaction rates.
29. How does the size of one Kelvin compare to one degree Celsius?
One Kelvin is exactly the same size as one degree Celsius. The scales have the same magnitude, they're just offset by 273.15 units.
30. How do you convert Kelvin to Celsius?
To convert Kelvin to Celsius, subtract 273.15 from the Kelvin temperature. The formula is: °C = K - 273.15.
31. What is the significance of 273.15 in the Celsius to Kelvin conversion?
273.15 is the difference between the zero points of the Celsius and Kelvin scales. It represents the Kelvin temperature at which water freezes (0°C).
32. How does the Celsius to Kelvin conversion affect calculations in chemistry?
Converting to Kelvin ensures that all temperatures are positive and proportional to the average kinetic energy of particles. This simplifies many thermodynamic calculations and equations in chemistry.
33. What's the boiling point of water in Kelvin?
The boiling point of water at standard pressure is 100°C, which converts to 373.15K (100 + 273.15).
34. What's the triple point of water in Kelvin and Celsius?
The triple point of water (where solid, liquid, and gas coexist) is defined as 273.16K, which is 0.01°C.
35. Why do some chemistry equations require temperature in Kelvin?
Many chemistry equations, especially those involving thermodynamics or kinetics, require Kelvin temperatures because they're based on absolute temperature scales. Using Celsius could lead to incorrect results or even mathematical impossibilities (like dividing by zero).
36. What's the difference between heat and temperature in relation to the Kelvin scale?
Heat is a form of energy transfer, while temperature (in Kelvin) is a measure of the average kinetic energy of particles. The Kelvin scale provides an absolute measure of this average energy, starting from zero at absolute zero.
37. Why is 0K theoretically impossible to reach?
According to the third law of thermodynamics, it's impossible to reach 0K because it would require removing all thermal energy from a system, which would violate the uncertainty principle of quantum mechanics.
38. How does the Kelvin scale relate to the concept of thermal expansion?
Thermal expansion is directly related to the absolute temperature. The Kelvin scale, starting at absolute zero, provides a direct relationship between temperature and the average kinetic energy of particles, which determines thermal expansion.
39. What's the significance of negative temperatures on the Celsius scale when converted to Kelvin?
Negative Celsius temperatures are still positive on the Kelvin scale. For example, -10°C is 263.15K. This illustrates that even at temperatures we consider "cold," particles still have significant thermal energy.
40. Why is the Kelvin scale considered more "fundamental" than the Celsius scale?
The Kelvin scale is considered more fundamental because it starts at absolute zero and directly relates to the energy of particles. It's also the SI unit for thermodynamic temperature, making it the standard for scientific measurements and calculations.
41. How does the concept of temperature relate to particle motion in the Kelvin scale?
In the Kelvin scale, temperature is directly proportional to the average kinetic energy of particles. As the Kelvin temperature increases, so does the average speed and energy of the particles in a substance.
42. What's the relationship between the Celsius to Kelvin conversion and the concept of heat capacity?
Heat capacity is the amount of heat required to raise the temperature of a substance by one degree. Because one Kelvin is the same size as one degree Celsius, the numerical value of heat capacity is the same in both scales, simplifying many calculations.
43. How does the Celsius to Kelvin conversion affect calculations in chemical kinetics?
Chemical kinetics often involves the Arrhenius equation, which relates reaction rates to temperature. This equation requires absolute temperature (Kelvin) to work correctly, as it involves an exponential term that wouldn't make sense with negative temperatures.
44. Why is it important to specify whether a temperature difference is in Celsius or Kelvin?
While a temperature difference of 1°C is the same as 1K, it's important to specify the scale when giving absolute temperatures. For example, 20K and 20°C are very different temperatures, but a change of 20K is the same as a change of 20°C.
45. What's the significance of the temperature 273.15K in chemistry?
273.15K is significant because it corresponds to 0°C, the freezing point of water at standard pressure. It's the offset between the Celsius and Kelvin scales and a crucial reference point in many chemical and physical processes.
46. Why is it incorrect to say "degrees Kelvin"?
The term "degrees Kelvin" is incorrect because Kelvin is an absolute scale defined without reference to any particular substance's behavior. The correct term is simply "Kelvin" or the symbol K, without the degree symbol.
47. How does the concept of absolute zero relate to the third law of thermodynamics?
The third law of thermodynamics states that the entropy of a perfect crystal at absolute zero (0K) is zero. This helps define the Kelvin scale and explains why absolute zero can be approached but never reached.
48. How does the Kelvin scale relate to the concept of black body radiation?
Black body radiation is directly related to the absolute temperature of an object. The Stefan-Boltzmann law, which describes the power radiated by a black body, uses temperature in Kelvin to the fourth power, emphasizing the importance of using an absolute scale.
49. Why is it important to use Kelvin in calculations involving the Maxwell-Boltzmann distribution?
The Maxwell-Boltzmann distribution, which describes the distribution of molecular speeds in a gas, requires absolute temperature (Kelvin). Using Kelvin ensures the distribution is always valid and correctly represents the range of molecular speeds at all temperatures.
50. How does the Celsius to Kelvin conversion affect calculations involving the Gibbs free energy?
The Gibbs free energy equation (ΔG = ΔH - TΔS) requires temperature in Kelvin. Using Celsius could lead to incorrect results, especially at low temperatures where Celsius values could be negative.
51. Why is it important to use Kelvin when calculating the efficiency of heat engines?
Heat engine efficiency calculations, based on the Carnot cycle, require absolute temperatures (Kelvin). Using Celsius could lead to nonsensical results, especially when the cold reservoir temperature is below 0°C.
52. What's the relationship between the Kelvin scale and the concept of absolute entropy?
Absolute entropy, as defined by the third law of thermodynamics, is based on the Kelvin scale. The entropy of a perfect crystal at 0K is defined as zero, providing a reference point for entropy calculations in thermodynamics.
53. Why is it important to use Kelvin in calculations involving the Clausius-Clapeyron equation?
The Clausius-Clapeyron equation, which relates vapor pressure to temperature, requires absolute temperature (Kelvin). Using Kelvin ensures the equation is valid at all temperatures and correctly represents phase equilibria.
54. How does the Celsius to Kelvin conversion affect calculations involving the Nernst equation in electrochemistry?
The Nernst equation, which relates the reduction potential of an electrochemical reaction to the standard electrode potential, requires temperature in Kelvin. Using Kelvin ensures the equation is valid at all temperatures and correctly represents the temperature dependence of electrochemical potentials.
55. What's the significance of using Kelvin in statistical thermodynamics calculations?
Statistical thermodynamics uses Kelvin because it directly relates to the energy of particles. Many equations in this field, such as those involving partition functions, require absolute temperature to correctly represent the distribution of energy states in a system.