Ion Definition - What is an Ion?, Explanation and FAQs
Ion definition
In this article we will discuss what is an ion, what is actual ion meaning, what are the examples of ion, how we define ion, what is ion in chemistry, what are the types of ion, then we will discuss what are the positively charged ions called, what are the negatively charged ions called and how ions are formed and more detailed description about an ion.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Ion definition
- What is an ion?
- What is an ion in chemistry?
- How are ions form?
- Ion ion bonding
What is an ion?
Here firstly we will discuss in general what is an ion. As we all know that in an atom there are two types of charged species electrons and protons and as electrons are more in number and revolve around the nucleus are conventionally considered to be of the negative nature and nucleus is considered of the positive nature and supposed to contain positive species called protons.
As already said that electrons are more in number and therefore outnumber the protons therefore there is some net charge on the atom and that net charge can also be due to some loss or gain of electrons in a molecule from the other atom. When this net charge is present on the atom it is called an ion. now as this is general ion meaning but now we will discuss what is the actual meaning of ion.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
What is an ion in chemistry?
So when we discuss ion meaning in general, we come to know that it is basically an atom or a molecule having a net electric charge on it due to some loss or gain of an electron. this gain or loss can be due to the formation of some required molecule and further in the formation of the compound. As we have discussed ion meaning now keeping that in mind we will quote some basic examples explaining the same.in chemistry we generally write the atomic symbols with their charges like sodium is written as Na+ and chlorine is written as Cl-.
From these examples, we can see that the sodium atom has a positive charge and the chlorine atom has a negative charge so from this we come to know that the positive ions are called a cation and the negative ions are called anions. So, from this, we concluded that there are two types of ions i.e that the positively charged ions are called a cation and the negatively charged ions are called anions.
The next question arises that how the ions are formed.
Related Topics Link, |
How are ions form?
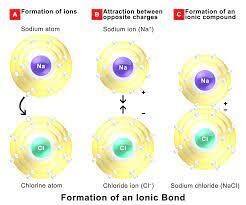
Talking about the atoms we know that in chemistry we have atoms and atoms are supposed to contain the proton which is positively charged and outside the nucleus contain the negatively charged electrons which balance each other effect and makes the entire atom neutral. In this, if the atom loses one electron then the charge becomes +1 and if the atom gains an electron then it attains -1 charge.
Also read :
- NCERT notes Class 11 Chemistry Chapter 7 Equilibrium
- NCERT solutions for Class 11 Chemistry Chapter 7 Equilibrium
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 7 Equilibrium
Ion ion bonding
As we all know that the chemistry shows different types of bonding between the atoms and such bonding differ depending upon the nature of the atom. as it is well known that opposites attract each other. So this is the main criteria we can say that is applied in the ionic bonding. We define ionic bonding in proper terms as the “ chemical bonding where the electrostatic force of attraction is seen among oppositely charged ions from two different blocks of the periodic table or between the atoms with sharply different electronegativities.
Also check-
Frequently Asked Questions (FAQs)
Atoms do so in order to achieve stable electronic configuration. Further to achieve the stable electronic configuration the valence shell electrons need to be completely filled or half-filled in order to achieve the stable electronic configuration. The list of ion examples is shown below:
|
These are a few ions examples commonly used.
Further talking about the atoms we come to know that the ions can be categorised as monoatomic ions and polyatomic ions. This can be explained as the ions consisting of a single atom are called the monoatomic ions and the ions consisting of two or more atoms are termed polyatomic ions. The process of formation of these polyatomic ions takes either during a chemical reaction. Generally, we see this through a very general experiment that was carried out by faraday. What faraday did, he only knew that when the metal is dissolved in a solution at one electrode and the new metal is formed at the other electrode. And then after discovering more about the nature of the electrodes he named the two electrodes that were dipped in the solution as anode and cathode and later from the naming of these electrodes the words anion and cation were introduced. From this experiment, a very interesting that arises here is that what makes the metal in the solution break and leads to the formation of the other metal on the second electrode? The answer to this question is that the breakdown of the ions and again the formation of the ions in order to form the polyatomic ions which are more stable than the previous one.
Here according to class 9, a polyatomic ion is a charged species composed of two or more atoms bonded by a covalent bond either from the two extreme sections of the periodic table or not much far away sections of the same periodic table or we can say that a metal complex considered to be acting as a single unit. Example of polyatomic ions SO42-, CO32-.
This can be better explained with the help of an example. When sodium (Na) and chlorine (Cl) are combined, the sodium atoms lose an electron forming a cation, and the chlorine atom being deficient in one electron to form an octet gains an electron in order to form an anion and then they get attached to each other form sodium chloride or what we say a common salt.
We should note here that the ionic bonds are always formed between the elements of the s block and the elements of the p block of the periodic table. These two block elements have a great electronegativity difference therefore they can easily form a bond.
We can here show the list of some of the ionic compounds so formed from the interaction between the ions having different or we can say opposite charges.
Sodium chloride | NaCl |
Potassium bromide | KBr |
Calcium iodide | CsI2 |
Cesium fluoride | CsF |
Lithium chloride | LiCl |
Magnesium nitride | Mg3N2 |
Cadmium sulphide | CdS |
These are some of the basic ionic compounds we usually use along with their symbols.
Properties of an ionic compound
Ionic compounds conduct electricity when in a liquid state but not in a solid state.
They have a reasonably higher melting point.
Ionic bonds are stronger so a large quantity of energy is needed to break the bond.
Ionic compounds show solubility in polar solvents, example water, but insoluble in non polar solvents like alcohol etc.
While writing an ion a symbol for the element is written followed by the superscript. The superscript is the charges on the ion followed by the + sign for cation and the – sign for an anion. No superscript is used for the neutral element which has a net charge of zero.
This is detailed information about ion definition. We have here discussed each and every aspect of this in detail.
Also Read
07 Feb'25 12:04 AM
07 Feb'25 12:00 AM
06 Feb'25 11:50 PM
06 Feb'25 11:48 PM
06 Feb'25 11:40 PM
09 Dec'24 11:40 AM
21 Oct'24 12:32 PM
21 Oct'24 11:57 AM
19 Oct'24 03:14 PM
19 Oct'24 03:08 PM

