Hydrocarbons - Notes, Topics, Formula, Books, FAQs
Hydrocarbons are chemical compounds composed of Carbon and Hydrogen. Everything mostly that we use in our daily life is hydrocarbons such as LPG, petrol, insecticides, soaps, etc. There is a large number of organic compounds that exist in nature for two reasons: Tetravalency and Catenation because of this property, hydrocarbons are able to exist in various forms like linear structure, branched-chain structure, and ring structure.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Important topics - Hydrocarbons
- Overview Of Hydrocarbons
- How to prepare for hydrocarbons
- Prescribed Books
The given below are the major applications of hydrocarbons:
(i) Natural Gas: This is naturally occurring hydrocarbon gas, consisting mainly of methane and some other higher alkanes as well. This gas in the form of LPG which mainly contains butane is mainly used in our kitchens.
(ii) Soaps And Detergents: Hydrocarbons are used in the manufacturing of soaps and detergents.
(iii) Insecticides And Pesticides: Hydrocarbons are majorly used in insecticides and pesticides. Some common examples of these containing hydrocarbons are DDT, Malathion, carbofuran, etc.
Important topics - Hydrocarbons
IUPAC Nomenclature Of Alkanes:
The simplest hydrocarbon is an alkane. It contains only carbon and hydrogen atoms single-bonded to one another. The general formula for the alkanes is CnH2n+2. These compounds will be named under the IUPAC nomenclature system with regard to the number of carbon atoms in the longest continuous chain. Names of alkanes end with the suffix "-ane."
Adsorption And Degree Of Unsaturation:
The phenomenon of the deposition of particles on the surface of the adsorbent is called Adsorption And Degree Of Unsaturation is calculated to analyze the total number of double bonds and rings in the organic compound. It is also known as the index of hydrogen deficiency or unsaturation index.
Preparation Of Alkanes:
Alkanes are also sometimes called paraffins and are defined as saturated hydrocarbons with the general formula CnH2n+2. Alkanes can be prepared by various methods such as hydrogenation of the alkenes, reduction of the alkyl halides, and decarboxylation of the carboxylic acids and their are many other preparations ofpreparations of alkanes.
Physical Properties Of Alkanes:
Alkanes normally have low melting and boiling points which are found to increase with increasing molecular weight. This can be further explained by the fact that the rate of increase in the size of alkanes is making more van der Wal forces operate on their increased surface areas. Also, it requires more energy for alkanes to undergo a state change.
Nomenclature And Isomerism Of Alkenes:
Alkenes are hydrocarbons that contain at least one carbon-carbon double bond. Their general formula is CnH2n Their names are uniform and clear due to the IUPAC system of nomenclature. Alkenes show two kinds of isomerism i.e., stereoisomerism and geometrical isomerism.
Preparation Of Alkenes:
Alkenes are hydrocarbons that contain at least one carbon-carbon double bond,
Hydroboration And Oxidation:
Hydroboration-oxidation is a two-step procedure for converting alkenes to alcohols. First is hydroboration: the reaction of an alkene with BH3 to give an organoborane intermediate. This rather unusual process takes place in syn-addition—that is, the boron and hydrogen atoms add to the same side of the double bond of the alkene. The addition is regioselective.
Preparation Of Alkynes:
Alkynes contain a carbon-carbon triple bond, C≡C. The simplest alkyne is ethyne, more commonly called acetylene, C₂H₂. Alkynes are prepared in many ways such as Dehydrohalogenation, Alkylation reaction, and various other methods for the Preparation Of Alkynes.
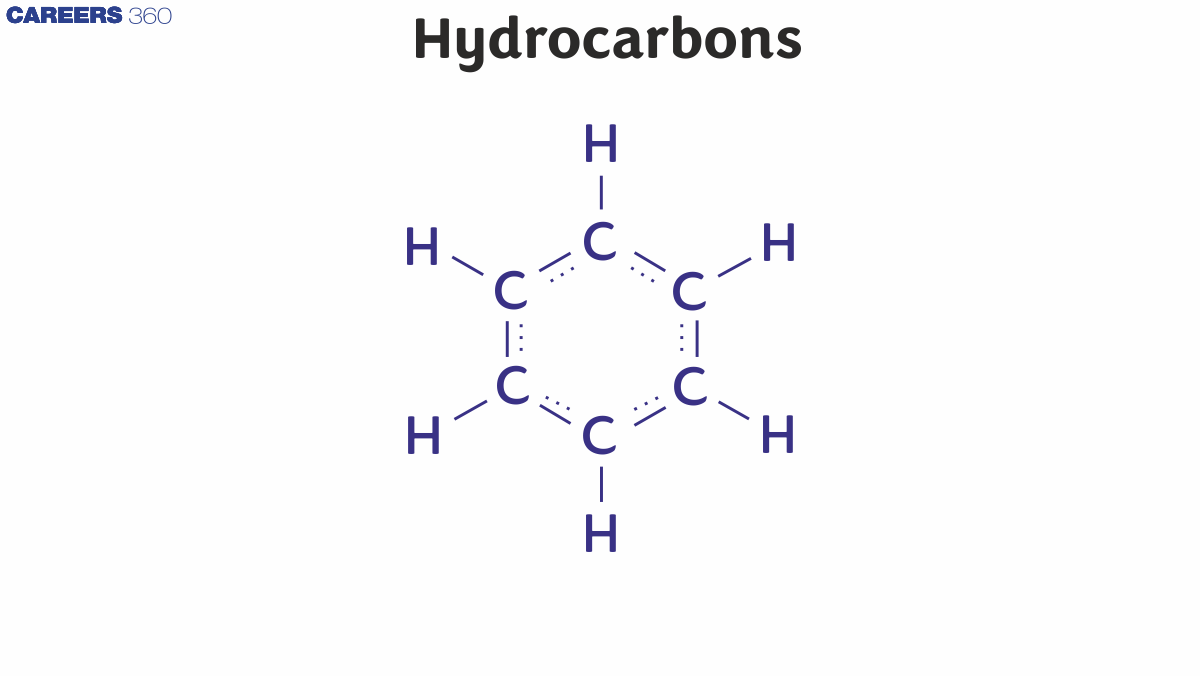
Aromaticity:
Aromaticity is defined as "An aromatic compound having a cyclic planar structure with (4n+2)π electrons and has high resonance energy and stability due to delocalization of pi electrons."
Electrophilic Substitution Reaction:
Electrophilic substitution reactions are a class of chemical reactions where some electrophile replaces a hydrogen atom in the aromatic ring. Such reactions are typical for the class of aromatic compounds, among which the most famous are benzene and its derivatives.
Also Read
- Chemical Properties Of Alkanes
- Conformation, Sawhorse, And Newman Projections
- Chemical Properties Of Alkenes
- Chemical Properties Of Alkynes
- Reaction Of Aromatic Compounds
- Friedel-crafts Reaction
- Benzene Reactions - Sulfonation, Nitration And Halogenation
- Reaction With Alkali
Related Topics,
Overview Of Hydrocarbons
The hydrocarbons can be classified in the following three categories, on the basis of the bonds present between carbon-carbon atoms.
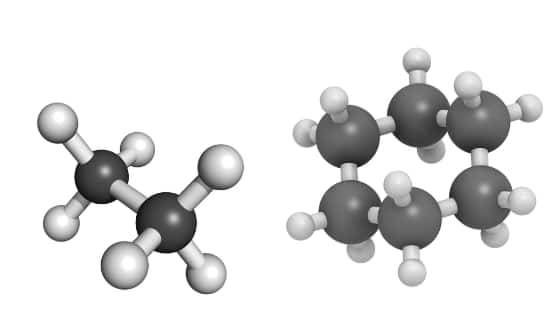
(i) Saturated Hydrocarbons: These are the types of hydrocarbons in which all carbon-carbon and carbon-hydrogen are singly bonded with each other. These hydrocarbons either exist as an open chain or circular ring structured and they are known as alkanes and cycloalkanes respectively. Some common examples of saturated hydrocarbons are:
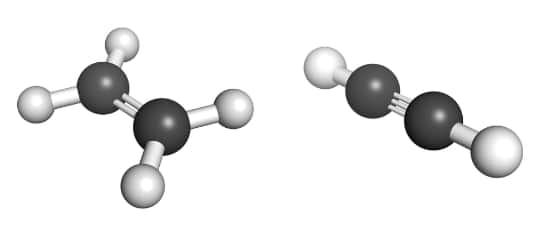
(ii) Unsaturated Hydrocarbon: These are the hydrocarbons that have double or triple bonds between the carbon atoms. These unsaturated hydrocarbons are of two types, i.e, Alkenes (containing double bonds) and Alkynes (containing triple bonds). The general formula for alkenes and alkynes are CnH2n and CnH2n-2, respectively.
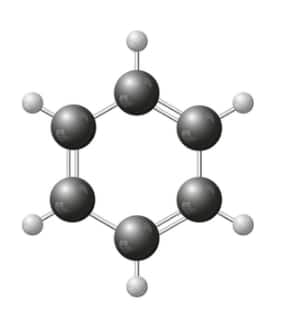
(iii) Aromatic Hydrocarbon: These hydrocarbons are circular rings with delocalized pi electrons between carbon atoms. The simplest aromatic compound known is benzene, with the chemical formula C6H6.
This classification of hydrocarbons can be tabulated as follows:
Hydrocarbon | Characteristic | Example |
Saturated (Alkanes) | Only a single bond | Butane |
Unsaturated (Alkenes, Alkynes) | Double and Triple bonds | Ethene, Butyne |
Aromatic | Localized pi electrons | Benzene |
Alkanes
Alkanes are the most basic class of organic compounds. They are also known as saturated compounds. All the carbon-carbon bonds in these molecules are single bonds.
- Nomenclature And Isomerism
The IUPAC rules for nomenclature and isomerism we have already studied in the previous chapter - 'Some basic principles of organic chemistry'. Here we will see them again with a few examples
The molecular formula of the compound is C4H10. Let's try to draw its different structures with their scientific names.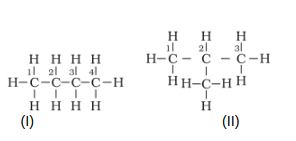
The (I) compound is named simply 'butane' and (II) compound is named as '2-Methylpropane'. Now since two molecules differ in their structures thus, they are known as structural isomers.
- Preparation
There are various methods to prepare alkanes. Some of them are listed below.
(i) From unsaturated hydrocarbons: In this reaction, alkenes are reacted with hydrogen in the presence of catalysts like palladium or nickel and form alkanes.
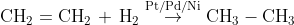 (ii) From alkyl halides: Alkyl halides on reaction with zinc and dilute hydrochloric acid give alkanes.
(ii) From alkyl halides: Alkyl halides on reaction with zinc and dilute hydrochloric acid give alkanes.
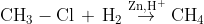
- Chemical Reactions
There are various important chemical reactions that are done by alkanes.
In this reaction, alkanes react with halogens and form alkyl halide as the product.
(i) Halogenation:
ii) Combustion: Alkanes undergo oxidation in the presence of air and form carbon dioxide and water.
Alkenes
Alkenes are another class of organic compounds. These compounds are also known as unsaturated hydrocarbons. In these compounds, one or more than one carbon-carbon double bond is always present.
- Nomenclature
The IUPAC rules for the nomenclature of alkenes have already been discussed in an earlier chapter. Some of the important alkenes and their IUPAC names are mentioned below.
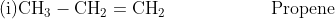
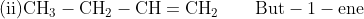
- Isomerism
Alkenes both show structural and geometrical isomerism.
(i) Structural Isomerism: The alkene compound with molecular formula C2H4 exists in various structures as shown below.


(ii) Geometrical Isomerism: Many of the alkenes show different geometries of the same molecule and isomers are known as cis and trans.

- Preparation
Alkenes are prepared in several ways. Some of the important methods to prepare alkenes are discussed below.
(i) From Alkynes: In this reaction, alkynes are reacted with hydrogen in the presence of palladised charcoal and form alkenes.
(ii) From Alkyl Halides: Alkyl halides on heating with alcoholic KOH form alkenes.
- Chemical Reactions
There are some important chemical reactions that alkenes do and form different products.
(i) Addition Of Halogens: In this reaction, halogens are added to alkenes and form vicinal halides as shown below.
(ii) Addition Of Alkyl Halides: In this reaction, hydrogen halide is reacted with an alkene and form alkyl halide.
How to prepare for hydrocarbons
- This chapter is the beginning of organic chemistry and possesses a very high weightage of marks in board exams and other competitive exams like JEE and NEET. It is completely theory-based. You are not supposed to memorize any formula. Practice a lot of numerical for getting a good hold on this chapter.
- To prepare for this chapter, firstly, you must have a basic understanding of the chapter - "Organic Chemistry- Some Basic Principles and Techniques". After this, you must read this chapter, "Hydrocarbons" from NCERT thoroughly with each and every example solved on your own.
- Practice each reaction carefully, especially, Grignard reagent, Wittig's reaction, Cope's reaction, etc. a lot of times, so that you would be able to understand the concepts at your fingertips.
- In a nutshell, it can be said that although this chapter is lengthier than other chapters, it is a very simple and straightforward one. So always say a "Big YES" to this chapter.
Prescribed Books
For this chapter, first, the NCERT book is best for initial-level preparation as well as for board exams. Now, after this, if you want to prepare for competitive exams like JEE and NEET, then these are the best books for you - Morrison and Boyd and R.K Gupta by Arihant publication. Meanwhile, in the preparation, you must continuously give the mock tests for the depth of knowledge. Our platform will help you with a variety of questions for deeper knowledge with the help of videos, articles, and mock tests.
Also Read,
Also Read
11 Mar'25 06:34 PM
11 Mar'25 06:27 PM
11 Mar'25 06:25 PM
11 Mar'25 05:54 PM
11 Mar'25 05:27 PM
05 Mar'25 05:23 PM
05 Mar'25 05:18 PM
05 Mar'25 04:59 PM
19 Feb'25 07:36 PM
19 Feb'25 07:33 PM
Articles
Questions related to
Correct Answer: Hydrocarbons of petroleum
Solution : The correct answer is Hydrocarbons of Petroleum.
Synthetic detergents are commonly used for efficient cleaning in various applications, including laundry, dishwashing and home cleaning. They are made using petroleum-based chemicals, specifically Alkylbenzene sulphonates and Alkyl sulfate, which are obtained from crude oil and chemically converted into surfactants, the essential cleaning agents in detergents.
Correct Answer: Compounds of carbon having double bonds or triple bonds between their carbon atoms are called unsaturated compounds.
Solution : The correct answer is Compounds of carbon having double bonds or triple bonds between their carbon atoms are called unsaturated compounds.
Unsaturated hydrocarbons are hydrocarbons (compounds consisting of hydrogen and carbon) that contain one or more multiple bonds, such as double bonds (C=C) or triple bonds (C≡C) between carbon atoms.
Correct Answer: Hermann Kolbe
Solution : The correct option is Hermann Kolbe.
The German chemist who used the technique of preparing hydrocarbons by electrolysis of solutions of salts of fatty acids was Hermann Kolbe. He made significant contributions to the field of organic chemistry, and his work on electrolysis paved the way for the synthesis of organic compounds from inorganic precursors. This process, known as Kolbe electrolysis, demonstrated that organic molecules could be created from inorganic sources, challenging the then-prevailing theory of vitalism in chemistry.

