1. What is the difference between slaked lime and quick lime?
Slaked lime is chemically calcium hydroxide (Ca(OH)2 also known as lime water and quick lime is calcium oxide (CaO).
2. What role does calcium play in bone health?
Calcium causes brittleness in bones. Getting adequate calcium in your diet not only keeps your bones strong, but it may also help you avoid hypertension.
3. Give two applications of quicklime?
Quicklime basic flux and utilised in sugar refining as well as disinfectants and germicides.
4. Is it true that calcium oxide dissolves in water?
Calcium oxide dissolves in glycerol and water.
5. What are the health hazards of quick lime?
Calcium oxide can harm the skin, nose, eyes, and respiratory system if it comes into contact with it.
6. How does the reactivity of calcium oxide compare to other s-block oxides?
Calcium oxide is more reactive than magnesium oxide but less reactive than sodium or potassium oxides. This trend follows the general increase in reactivity down the s-block, with some exceptions due to factors like ionic size and charge density.
7. How does the basicity of calcium oxide compare to other metal oxides?
Calcium oxide is a strong base, more basic than most transition metal oxides but less basic than Group 1 metal oxides. Its basicity arises from the ability of the oxide ion to accept protons in aqueous solutions.
8. How does the solubility of calcium oxide in water change with temperature?
The solubility of calcium oxide in water decreases as temperature increases, which is opposite to the trend for most solids. This unusual behavior is due to the exothermic nature of its dissolution process.
9. How does the reaction of calcium oxide with carbon dioxide differ from its reaction with water?
With carbon dioxide, calcium oxide forms calcium carbonate (CaCO3) in a slow reaction that absorbs CO2. With water, it rapidly forms calcium hydroxide (Ca(OH)2) in an exothermic reaction. The CO2 reaction is reversible, while the water reaction is essentially irreversible under normal conditions.
10. What is calcium oxide and why is it called quicklime?
Calcium oxide (CaO) is a white, alkaline compound formed by heating calcium carbonate. It's called quicklime because of its rapid reaction with water, which produces heat and calcium hydroxide.
11. Why is calcium oxide sometimes referred to as burnt lime?
Calcium oxide is called burnt lime because it's produced by burning (calcining) limestone (calcium carbonate) at high temperatures. This process drives off carbon dioxide, leaving behind calcium oxide, hence the term "burnt" lime.
12. How does the thermal decomposition of calcium carbonate to form calcium oxide demonstrate Le Chatelier's principle?
The thermal decomposition of calcium carbonate to calcium oxide (CaCO3 → CaO + CO2) is an equilibrium reaction. According to Le Chatelier's principle, increasing temperature shifts the equilibrium to the right, favoring CaO formation. Removing CO2 also drives the reaction forward, demonstrating how changes in conditions affect chemical equilibria.
13. What is the environmental impact of calcium oxide production?
The production of calcium oxide from limestone releases significant amounts of CO2, contributing to greenhouse gas emissions. However, some of this CO2 is reabsorbed when CaO is used in certain applications, like cement setting. The energy-intensive production process also has environmental implications.
14. What is the role of calcium oxide in the production of calcium metal?
Calcium oxide is a key intermediate in the production of calcium metal. It's first reduced to calcium by aluminum at high temperatures in a vacuum (the Pidgeon process). The high stability of CaO makes this process challenging, requiring specific conditions to overcome the strong Ca-O bond.
15. What is the role of calcium oxide in the production of calcium carbide?
Calcium oxide is a key reactant in producing calcium carbide (CaC2). It's heated with carbon (coke) in an electric arc furnace to form calcium carbide and carbon monoxide. This reaction is important in the production of acetylene gas.
16. Why does calcium oxide have a high melting point?
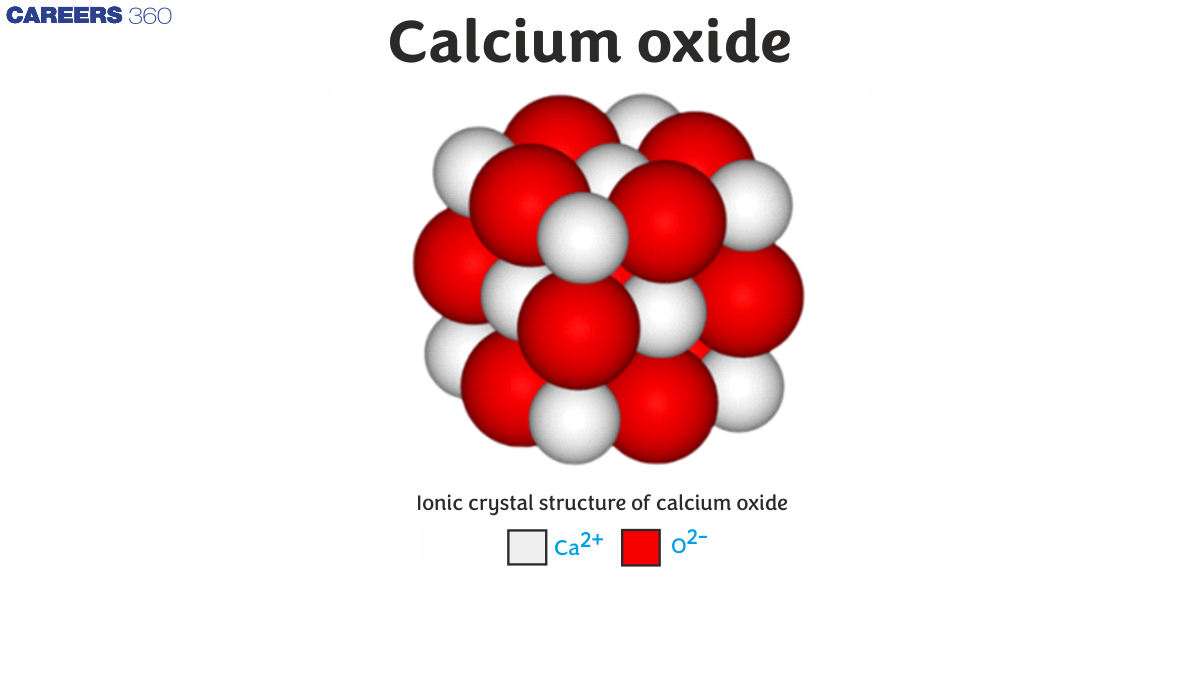
Calcium oxide has a high melting point (2,572°C) due to the strong ionic bonds between Ca2+ and O2- ions in its crystal lattice. These bonds require significant energy to break, resulting in a high melting point.
17. How does the electron configuration of calcium contribute to the properties of calcium oxide?
Calcium's electron configuration ([Ar]4s2) allows it to easily lose two electrons, forming Ca2+ ions. This leads to the formation of strong ionic bonds with oxide ions (O2-) in calcium oxide, contributing to its high melting point and other properties.
18. How does the ionic radius of calcium affect the properties of calcium oxide?
The relatively large ionic radius of Ca2+ compared to other divalent cations affects the crystal structure and reactivity of calcium oxide. It results in a less dense packing of ions, contributing to properties like its high reactivity with water and its effectiveness as a base.
19. What is the significance of calcium oxide's hygroscopic nature?
Calcium oxide's hygroscopic nature means it readily absorbs moisture from the air. This property makes it useful as a desiccant but also means it must be stored carefully to prevent unwanted reactions with atmospheric moisture.
20. What is the difference between calcium oxide and slaked lime?
Calcium oxide (CaO) is quicklime, while slaked lime is calcium hydroxide (Ca(OH)2). Slaked lime is produced when calcium oxide reacts with water. They have different chemical properties and uses, though both are alkaline.
21. How does the structure of calcium oxide contribute to its reactivity?
Calcium oxide has an ionic crystal structure with strong Ca2+-O2- bonds. This structure allows for quick dissociation in water, contributing to its high reactivity and ability to form calcium hydroxide rapidly.
22. How does the crystal structure of calcium oxide influence its properties?
Calcium oxide has a face-centered cubic crystal structure, similar to sodium chloride. This structure, with its strong ionic bonds, contributes to properties like high melting point, brittleness, and its ability to conduct electricity when molten but not in solid form.
23. How does calcium oxide contribute to the setting of cement?
In cement, calcium oxide reacts with water to form calcium hydroxide, which then reacts with silicates to form calcium silicate hydrate. This compound is responsible for the strength and hardening of cement, making CaO crucial in the cement-setting process.
24. What is the significance of calcium oxide's high enthalpy of formation?
The high enthalpy of formation of calcium oxide (-635 kJ/mol) indicates that it's a very stable compound. This stability contributes to its usefulness in high-temperature applications and its role as a strong base. It also means that significant energy is required to decompose CaO back into its elements.
25. How does the presence of impurities affect the properties of calcium oxide?
Impurities in calcium oxide can significantly affect its properties. For example, magnesium impurities can alter its reactivity and affect its performance in applications like cement production. Iron impurities can affect the color and purity of the product, which is important in applications requiring high-purity CaO.
26. Why is calcium oxide considered a refractory material?
Calcium oxide is considered a refractory material due to its high melting point and stability at elevated temperatures. These properties make it useful in lining furnaces and kilns in various industrial processes.
27. What role does calcium oxide play in the lime cycle?
Calcium oxide is a key component in the lime cycle. It's produced by heating calcium carbonate (limestone) and can be converted back to calcium carbonate by reacting with carbon dioxide. This cycle is important in many industrial processes and in nature.
28. How does calcium oxide contribute to air pollution control?
Calcium oxide is used in flue gas desulfurization to remove sulfur dioxide from exhaust gases in power plants. It reacts with SO2 to form calcium sulfite, which can be oxidized to gypsum, helping reduce acid rain and air pollution.
29. Why is calcium oxide used in steelmaking?
Calcium oxide is used in steelmaking as a flux. It helps remove impurities like silica and phosphorus from iron ore by forming slag. The basic nature of CaO also helps neutralize acidic impurities in the steel-making process.
30. What happens when calcium oxide reacts with water, and why is this reaction important?
When calcium oxide reacts with water, it forms calcium hydroxide (Ca(OH)2) in an exothermic reaction. This reaction is important in construction (for making slaked lime) and in neutralizing acidic soils in agriculture.
31. How does calcium oxide affect soil pH, and why is this important in agriculture?
Calcium oxide increases soil pH by neutralizing acidity. When added to soil, it reacts with water to form calcium hydroxide, which then dissociates to release hydroxide ions. This pH increase is important for optimizing nutrient availability and promoting healthy plant growth in acidic soils.
32. Why is calcium oxide sometimes used in waste water treatment?
Calcium oxide is used in wastewater treatment to adjust pH, remove phosphates, and help in the precipitation of heavy metals. Its ability to form insoluble compounds with many contaminants makes it effective in purifying water.
33. Why is calcium oxide used in the production of glass?
Calcium oxide is used in glass production as a flux and stabilizer. It lowers the melting point of silica, improves the workability of molten glass, and enhances the chemical durability of the final product. It also helps prevent devitrification (crystallization) of the glass.
34. How does calcium oxide participate in the Solvay process?
In the Solvay process, used to produce sodium carbonate, calcium oxide plays a crucial role. It's used to regenerate ammonia from ammonium chloride, a byproduct of the process. This helps make the Solvay process more economically viable by recycling a key component.
35. How does calcium oxide react with acids, and why is this reaction important?
Calcium oxide reacts vigorously with acids to form calcium salts and water. This neutralization reaction is exothermic and is important in applications like controlling soil acidity, neutralizing industrial waste, and in some chemical manufacturing processes.
36. What is the role of calcium oxide in the production of biodiesel?
Calcium oxide can be used as a heterogeneous catalyst in the transesterification reaction to produce biodiesel. Its basic nature promotes the reaction between triglycerides and methanol, forming fatty acid methyl esters (biodiesel) and glycerol.
37. Why is calcium oxide sometimes used in food preservation?
Calcium oxide is used in food preservation due to its ability to absorb moisture and carbon dioxide. It can help maintain a dry environment in packaged foods, preventing spoilage. However, it's not added directly to food but used in packaging materials.
38. How does the reactivity of calcium oxide with halogens compare to that of other s-block elements?
Calcium oxide reacts with halogens to form calcium halides, but less vigorously than Group 1 oxides. The reaction follows the trend of increasing reactivity down the group, with CaO being more reactive than MgO but less than SrO or BaO. This trend is related to the decreasing lattice energy and increasing basic strength down the group.
39. How does calcium oxide contribute to the self-healing properties of some concretes?
In some advanced concretes, unreacted calcium oxide particles can contribute to self-healing properties. When cracks form and expose these particles to water, they hydrate and expand, potentially filling small cracks. This process can help extend the lifespan of concrete structures.
40. Why is calcium oxide used in the production of paper?
In paper production, calcium oxide is used in the kraft process to regenerate sodium hydroxide from sodium carbonate. This helps in maintaining the alkalinity of the pulping liquor and in recovering chemicals, making the process more economical and environmentally friendly.
41. How does calcium oxide interact with carbon monoxide, and why is this interaction important?
At high temperatures, calcium oxide can react with carbon monoxide to form calcium and carbon dioxide (CaO + CO → Ca + CO2). This reaction is the basis of the carbothermal reduction process, which is important in metallurgy for extracting some metals from their oxides.
42. Why is calcium oxide sometimes used in archaeological preservation?
Calcium oxide is used in archaeological preservation due to its ability to absorb moisture and carbon dioxide. When applied to limestone artifacts, it can help prevent further degradation by forming a protective layer of calcium carbonate on the surface.
43. How does the solubility product of calcium oxide compare to other alkaline earth metal oxides?
The solubility product of calcium oxide is higher than that of magnesium oxide but lower than strontium or barium oxides. This trend reflects the general increase in solubility down the group, which is related to the decreasing lattice energy and increasing ionic character of the metal-oxygen bond.
44. What is the significance of calcium oxide's ability to form solid solutions?
Calcium oxide can form solid solutions with other alkaline earth metal oxides and some transition metal oxides. This property is important in materials science and ceramics, as it allows for the tuning of properties like electrical conductivity, thermal expansion, and catalytic activity.
45. How does calcium oxide contribute to the removal of sulfur from petroleum products?
Calcium oxide is used in the desulfurization of petroleum products. It reacts with sulfur compounds to form calcium sulfide or calcium sulfate, effectively removing sulfur from the fuel. This process is important for reducing sulfur dioxide emissions when the fuel is burned.
46. Why is calcium oxide sometimes used in the tanning of leather?
In leather tanning, calcium oxide is used to remove hair and fat from animal hides. It creates an alkaline environment that helps break down proteins in the hair follicles and swells the hide, preparing it for the tanning process. This use demonstrates CaO's strong basic properties and its ability to break down organic matter.
47. How does the presence of calcium oxide affect the melting point of slag in metallurgical processes?
Calcium oxide acts as a flux in metallurgical processes, lowering the melting point of slag. It breaks up the network structure of silicate minerals, reducing the viscosity of the molten slag. This makes the slag more fluid, helping to separate impurities from the metal more effectively.
48. What is the role of calcium oxide in the production of calcium cyanamide?
Calcium oxide is a key reactant in producing calcium cyanamide (CaCN2), an important fertilizer and industrial chemical. CaO is reacted with carbon and nitrogen at high temperatures to form calcium cyanamide. This process, known as the Frank-Caro process, demonstrates CaO's versatility in industrial chemistry.
49. How does calcium oxide contribute to the strength development in geopolymer concretes?
In geopolymer concretes, which are alternatives to traditional Portland cement, calcium oxide can enhance strength development. It participates in the geopolymerization reaction, forming calcium silicate hydrate and calcium aluminate hydrate phases that contribute to the material's strength and durability.
50. Why is calcium oxide sometimes used in the production of hydrogen gas?
Calcium oxide can be used in the production of hydrogen gas through the calcium oxide-steam process. In this method, CaO reacts with water at high temperatures to produce calcium hydroxide and hydrogen gas. This process is being explored as a potential method for clean hydrogen production.
51. How does the presence of calcium oxide affect the optical properties of glass?
Calcium oxide in glass affects its optical properties by influencing the refractive index and dispersion. It also improves the chemical durability of the glass, making it more resistant to weathering. The amount of CaO can be adjusted to fine-tune these properties for specific applications in optics and glassmaking.
52. What is the significance of calcium oxide's role in the capture and storage of carbon dioxide?
Calcium oxide is being investigated for carbon capture and storage due to its ability to react with CO2 to form calcium carbonate. This process, known as mineral carbonation, could potentially be used to sequester large amounts of CO2, helping to mitigate climate change. The reversibility of this reaction at high temperatures also makes it interesting for CO2 capture and release cycles.
53. How does calcium oxide participate in the Ostwald process for nitric acid production?
In the Ostwald process for nitric acid production, calcium oxide plays an indirect but important role. It's used to produce calcium nitrate, which is then thermally decomposed to yield nitrogen dioxide, a key intermediate in nitric acid synthesis. This demonstrates CaO's versatility in industrial chemical processes.
54. Why is the purity of calcium oxide important in certain applications?
The purity of calcium oxide is crucial in applications like high-quality glass production, certain chemical syntheses, and in the food industry. Impurities can affect reactivity, introduce unwanted color, or contaminate the final product. High-purity CaO is often required to ensure consistent performance and meet stringent quality standards.
55. How does the particle size of calcium oxide affect its reactivity and applications?
The particle size of calcium oxide significantly affects its reactivity and applications. Smaller particles have a larger surface area-to-volume ratio, leading to faster reaction rates and more complete reactions. This is particularly important in applications like flue gas desulfurization, where rapid reaction with sulfur dioxide is crucial. However, very fine particles can be more challenging to handle and store due to their increased reactivity with atmospheric moisture and CO2.