1. What is the Aufbau Principle?
The Aufbau Principle is a rule that states electrons fill atomic orbitals from the lowest to highest energy levels.
2. What is the Aufbau Principle?
The Aufbau Principle is a rule in chemistry that describes how electrons are added to atomic orbitals as an atom's atomic number increases. It states that electrons fill orbitals from lowest energy to highest energy, following the n + l rule.
3. What is the general order of filling according to the Aufbau Principle?
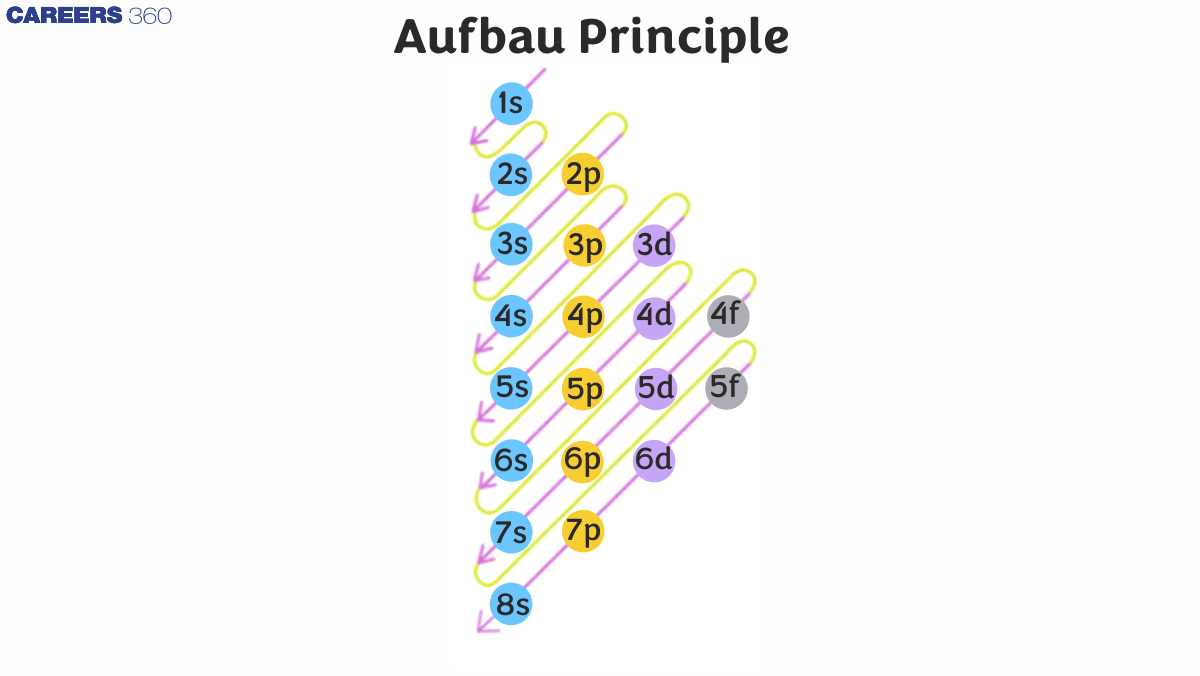
The general order of filling is as follows:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d → 5p → 6s → 4f → 5d → 6p, and so forth. This order follows the relative energy levels of each orbital.
4. What does the l denotes in the n+l rule?
The quantum numbers "n" and "l" in the (n + l) rule are used to specify the state of a specific electron orbital in an atom. Here, l denotes the angular momentum quantum number, which is related to the orbital's form.
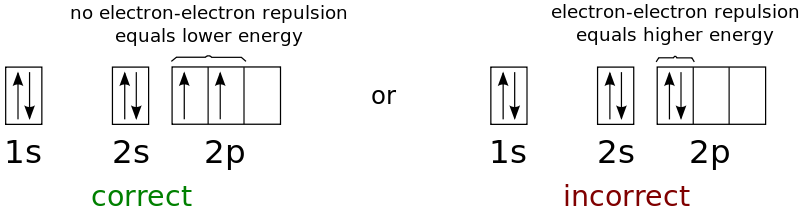
5. State Hund’s Rule?
It states that before any orbital in a subshell is doubly occupied, it is singly occupied with one electron, and all electrons in singly occupied orbitals have the same spin.
6. Are there any exceptions to the Aufbau Principle?
Yes, there are exceptions to the Aufbau Principle, particularly for some transition and inner transition elements. For example, copper (Cu) and chromium (Cr) have electron configurations that don't strictly follow the principle due to the stability of half-filled or fully-filled d-orbitals.
7. Can you explain how the Aufbau Principle applies to the lanthanide and actinide series?
For lanthanides and actinides, the Aufbau Principle shows that f-orbitals are filled. In lanthanides, 4f orbitals are filled after 6s but before 5d. In actinides, 5f orbitals are filled. This explains the similar chemical properties within each series.
8. Can you explain how the Aufbau Principle applies to transition elements?
For transition elements, the Aufbau Principle shows that the d-orbitals begin to fill after the 4s orbital. This explains why transition elements have similar outer electron configurations and why their chemical properties are often determined by their d-electrons.
9. Can you explain how the Aufbau Principle applies to anions and cations?
For anions and cations, the Aufbau Principle is applied in reverse. When forming a cation, electrons are removed from the highest energy orbitals first. For anions, electrons are added to the lowest energy available orbitals.
10. Why is it important to understand the Aufbau Principle in chemistry?
Understanding the Aufbau Principle is crucial in chemistry because it forms the basis for electron configuration, which in turn determines an element's chemical and physical properties, reactivity, and behavior in chemical bonds.
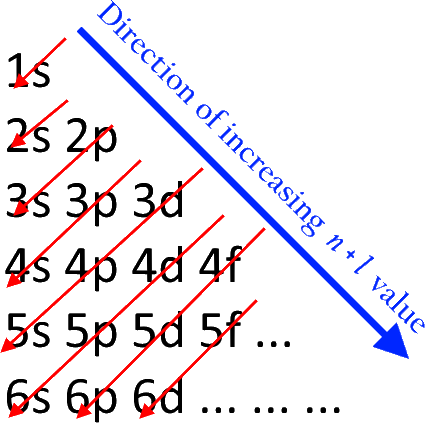
11. What is the n + l rule in the Aufbau Principle?
The n + l rule, also known as the Madelung rule, determines the order of orbital filling. In this rule, 'n' is the principal quantum number, and 'l' is the azimuthal quantum number. Orbitals with lower n + l values are filled first.
12. Can you explain the concept of "building up" in the Aufbau Principle?
The concept of "building up" in the Aufbau Principle refers to the process of adding electrons one by one to an atom, starting from the simplest atom (hydrogen) and progressing to more complex atoms. This process follows a specific order based on energy levels and sublevels.
13. Who proposed the Aufbau Principle?
The Aufbau Principle was proposed by Niels Bohr in 1920. However, the term "Aufbau" (meaning "building up" in German) was later coined by Erwin Schrödinger.
14. What role do quantum numbers play in the Aufbau Principle?
Quantum numbers are crucial in the Aufbau Principle as they define the energy levels, sublevels, and orbitals where electrons are placed. The principal quantum number (n) and azimuthal quantum number (l) are particularly important in determining the order of orbital filling.
15. How does the Aufbau Principle explain the formation of electron shells?
The Aufbau Principle explains the formation of electron shells by showing how electrons fill orbitals in order of increasing energy. Each new shell corresponds to a new principal quantum number (n), and the principle dictates how these shells are populated.
16. What is the significance of "aufbau" diagrams in learning electron configurations?
Aufbau diagrams, also known as electron configuration diagrams, visually represent how electrons fill orbitals according to the Aufbau Principle. They help students understand and remember the order of orbital filling and electron distribution.
17. What is the connection between the Aufbau Principle and atomic orbitals?
The Aufbau Principle directly relates to atomic orbitals by specifying the order in which these orbitals are filled with electrons. It takes into account the energy and spatial arrangement of different orbitals (s, p, d, f) within each energy level.
18. What does "Aufbau" mean in German?
"Aufbau" is a German word that means "building up" or "construction." In the context of atomic structure, it refers to the process of building up electron configurations.
19. How does the Aufbau Principle relate to electron configuration?
The Aufbau Principle guides the process of writing electron configurations. It determines the order in which electrons fill orbitals, starting from the lowest energy level and progressing to higher energy levels.
20. Why do electrons fill lower energy orbitals first?
Electrons fill lower energy orbitals first because it's energetically favorable. This arrangement results in the most stable electron configuration for the atom.
21. What is the correct order of orbital filling according to the Aufbau Principle?
The correct order of orbital filling is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p.
22. How does the Pauli Exclusion Principle relate to the Aufbau Principle?
The Pauli Exclusion Principle complements the Aufbau Principle by stating that no two electrons in an atom can have the same set of quantum numbers. This principle limits the number of electrons that can occupy each orbital to two, with opposite spins.
23. How does the Aufbau Principle relate to the concept of electron shielding?
The Aufbau Principle relates to electron shielding by showing how inner electrons shield outer electrons from the full nuclear charge. As electrons are added according to the principle, the effect of shielding on outer electrons becomes more pronounced.
24. How does the Aufbau Principle explain the formation of chemical bonds?
The Aufbau Principle explains chemical bond formation by showing how atoms achieve stable electron configurations. Atoms often form bonds to attain filled outer shells, either by sharing, gaining, or losing electrons as predicted by their electron configurations.
25. How does the Aufbau Principle help in understanding the concept of electronegativity?
The Aufbau Principle aids in understanding electronegativity by showing how electron configurations change across the periodic table. Elements with nearly filled outer shells (as determined by the principle) tend to have higher electronegativity.
26. What is the relationship between the Aufbau Principle and atomic emission spectra?
The Aufbau Principle relates to atomic emission spectra by determining the ground state electron configuration. When excited electrons return to lower energy levels, they emit light of specific wavelengths, creating characteristic emission spectra for each element.
27. How does the Aufbau Principle explain the formation of molecular orbitals?
While the Aufbau Principle primarily deals with atomic orbitals, it provides the foundation for understanding molecular orbital theory. The atomic orbitals, filled according to the principle, combine to form molecular orbitals in a similar energy-based order.
28. How does the Aufbau Principle help in predicting the reactivity of elements?
The Aufbau Principle helps predict reactivity by determining the number and arrangement of valence electrons. Elements with nearly filled or half-filled shells (as determined by the principle) often exhibit unique reactivity due to their electron configurations.
29. How does the Aufbau Principle relate to the concept of electron spin?
While the Aufbau Principle primarily deals with orbital filling, it works in conjunction with the Pauli Exclusion Principle, which introduces the concept of electron spin. Each orbital can hold two electrons with opposite spins, adhering to both principles.
30. Can you explain how the Aufbau Principle applies to the formation of ionic compounds?
The Aufbau Principle helps explain ionic compound formation by showing how atoms can achieve stable electron configurations. Metals tend to lose electrons to reach a noble gas configuration, while non-metals gain electrons, as predicted by their electron structures.
31. How does the Aufbau Principle relate to the Periodic Table?
The Aufbau Principle explains the structure of the Periodic Table. As you move across a period, electrons are added to the same energy level. When you start a new row, electrons begin filling the next energy level.
32. How does the Aufbau Principle help in predicting chemical properties?
The Aufbau Principle helps predict chemical properties by determining an element's electron configuration. This configuration influences properties such as valency, reactivity, and bonding behavior.
33. What is the relationship between the Aufbau Principle and Hund's rule?
The Aufbau Principle works in conjunction with Hund's rule. While the Aufbau Principle determines which orbitals are filled, Hund's rule specifies how electrons are distributed within a set of degenerate orbitals.
34. What is the significance of the 2n² rule in relation to the Aufbau Principle?
The 2n² rule relates to the Aufbau Principle by determining the maximum number of electrons in each shell. According to this rule, each shell can hold up to 2n² electrons, where n is the principal quantum number of the shell.
35. How does the Aufbau Principle account for the stability of noble gas configurations?
The Aufbau Principle helps explain the stability of noble gas configurations by showing that these elements have completely filled outer shells. This full outer shell configuration is energetically favorable and contributes to the chemical inertness of noble gases.
36. How does the Aufbau Principle explain the concept of valence electrons?
The Aufbau Principle helps identify valence electrons by showing which electrons occupy the outermost shell of an atom. These are typically the electrons in the highest energy level, which are most involved in chemical bonding and reactions.
37. What is the relationship between the Aufbau Principle and ionization energy?
The Aufbau Principle relates to ionization energy by explaining the electron configuration of atoms. Generally, electrons in higher energy levels (as determined by the Aufbau Principle) require less energy to remove, influencing the ionization energy trends across the periodic table.
38. How does the Aufbau Principle explain the formation of subshells?
The Aufbau Principle explains subshell formation by showing how electrons fill orbitals within each main energy level. Subshells (s, p, d, f) correspond to different azimuthal quantum numbers and are filled in a specific order based on their energy.
39. Why is the 4s orbital filled before the 3d orbital according to the Aufbau Principle?
The 4s orbital is filled before the 3d orbital because it has a slightly lower energy. This is due to the interplay between the principal quantum number (n) and the effective nuclear charge experienced by the electrons in these orbitals.
40. How does the Aufbau Principle help in understanding periodic trends?
The Aufbau Principle helps explain periodic trends by showing how electron configurations change across the periodic table. This influences trends in atomic radius, ionization energy, electron affinity, and electronegativity.
41. How does the Aufbau Principle explain the difference between core and valence electrons?
The Aufbau Principle distinguishes between core and valence electrons by showing how electrons fill inner shells completely before moving to outer shells. Core electrons are those in fully filled inner shells, while valence electrons occupy the outermost, partially filled shell.
42. How does the Aufbau Principle help in predicting the magnetic properties of atoms?
The Aufbau Principle, along with Hund's rule, helps predict magnetic properties by showing how unpaired electrons are distributed in an atom. The number of unpaired electrons, determined by the electron configuration, influences an atom's magnetic behavior.
43. How does the Aufbau Principle relate to the concept of effective nuclear charge?
The Aufbau Principle relates to effective nuclear charge by showing how electron shielding increases as more electrons are added to an atom. This affects the attraction between the nucleus and outer electrons, influencing atomic properties.
44. How does the Aufbau Principle help in understanding atomic size trends?
The Aufbau Principle helps explain atomic size trends by showing how electron shells are filled. As new shells are added, the atomic radius generally increases. However, the principle also explains exceptions due to factors like increased nuclear charge.
45. What is the relationship between the Aufbau Principle and electron affinity?
The Aufbau Principle relates to electron affinity by determining the electron configuration of atoms. Elements with nearly filled shells (as determined by the principle) often have high electron affinities, as they can achieve stable configurations by gaining electrons.
46. Can you explain how the Aufbau Principle applies to excited state electron configurations?
For excited state configurations, the Aufbau Principle is temporarily violated. Electrons can be promoted to higher energy orbitals, deviating from the ground state configuration. However, these excited states are unstable and tend to return to the ground state.
47. What is the significance of the Aufbau Principle in spectroscopy?
In spectroscopy, the Aufbau Principle helps predict and interpret atomic spectra. The principle explains the ground state configuration from which electrons can be excited, determining the possible electronic transitions and resulting spectral lines.
48. How does the Aufbau Principle relate to the concept of hybridization?
While the Aufbau Principle determines the ground state electron configuration, hybridization explains how these orbitals can mix to form new hybrid orbitals. Understanding the initial configuration is crucial for predicting possible hybridization states.
49. Can you explain how the Aufbau Principle applies to multi-electron atoms?
For multi-electron atoms, the Aufbau Principle becomes more complex. It accounts for electron-electron repulsions and the increasing nuclear charge, which affect the energy levels of orbitals. This explains why the actual order of orbital filling can sometimes deviate from the simple n + l rule.
50. How does the Aufbau Principle help in understanding the periodic law?
The Aufbau Principle supports the periodic law by explaining why elements in the same group have similar chemical properties. It shows that elements in a group have the same outer electron configuration, leading to similar chemical behavior.
51. Can you explain how the Aufbau Principle relates to the concept of electron promotion?
The Aufbau Principle provides the ground state configuration from which electron promotion can occur. In some cases, particularly in bonding situations, electrons can be promoted to higher energy orbitals, temporarily deviating from the Aufbau arrangement.
52. What is the significance of the Aufbau Principle in understanding oxidation states?
The Aufbau Principle is crucial for understanding oxidation states as it determines the electron configuration of atoms. The number of valence electrons, as predicted by the principle, influences the possible oxidation states an element can adopt in compounds.
53. How does the Aufbau Principle contribute to our understanding of chemical periodicity?
The Aufbau Principle is fundamental to understanding chemical periodicity. It explains why elements in the same group have similar properties and why there are trends in properties across periods, based on the systematic filling of electron shells and subshells.
54. What role does the Aufbau Principle play in quantum chemistry calculations?
In quantum chemistry calculations, the Aufbau Principle provides the starting point for more complex electronic structure calculations. It offers the initial electron configuration from which more sophisticated models can be built to accurately describe atomic and molecular systems.