First 20 Elements of the Periodic Table: Names, Symbols & Atomic Numbers
The first 20 elements of the Periodic table form the core of Chemistry. Every element in the periodic table is arranged based on its chemical and physical properties, such as atomic number, electronic configuration, and recurring chemical properties. These are the very basic elements, but they form the basis for the entire chemistry.
These elements represent the wide range of Metals, Non-metals, and Noble Gases. From the importance of Carbon in Organic life to the essential role of Calcium in bones and teeth, these elements play an important role in everyday life. Inside the periodic table , elements are arranged in the form of rows and columns. The rows are called periods, and the columns are called groups. The basic understanding of these elements helps to build a strong foundation in Chemistry.
Atomic Numbers and Symbols of the First 20 Elements
|
Atomic numbers |
Element |
Symbol |
|
|
Hydrogen |
H |
|
|
Helium |
He |
|
|
Lithium |
Li |
|
|
Berilliyum |
Be |
|
|
Boron |
B |
|
|
Carbon |
C |
|
|
Nitrogen |
N |
|
|
Oxygen |
O |
|
|
Fluorine |
F |
|
|
Neon |
Ne |
|
|
Sodium |
Na |
|
|
Magnesium |
Mg |
|
|
Aluminium |
Al |
|
|
Silicon |
Si |
|
|
Phosphorous |
P |
|
|
Sulfur |
S |
|
|
Chlorine |
Cl |
|
|
Argon |
Ar |
|
|
Potassium |
K |
|
|
calcium |
Ca |
An in-depth look at the first 20 elements in the periodic table is useful. Six of these elements make up nearly all of the mass of the human body. The first 20 elements provide an excellent overview of the various element groups. They can also be found in more common chemical processes. The elements are given in ascending atomic numbers from 1 to 20 order. The atomic number refers to the number of protons in each element's atoms.
Also read -
First 20 elements Of The Periodic Table
The periodic table is the representation of elements in the increasing order of their atomic number.
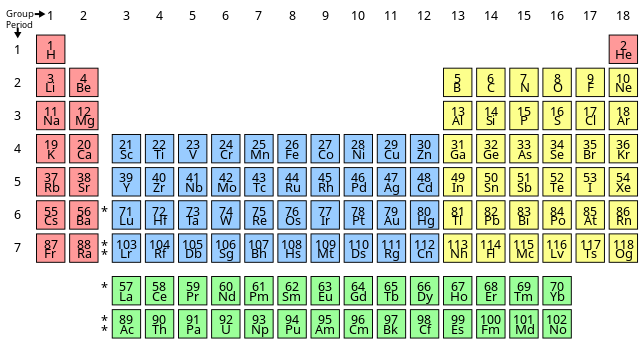
Name and Symbols of Elements
The atomic number, element name, or element symbol can all be used to identify elements. A one- or two-letter abbreviation of the name serves as the emblem. Some symbols, on the other hand, allude to old element names. The symbol for sodium, for given as Na. This is a reference to the Latin word natrium, which was once used to refer to caustic soda. In Latin, the name of the atomic no of sodium is found to be atrium. Potassium's symbol is K, which is derived from the Latin word kalium, which denoted alkali or potash. An element symbol's initial letter is capitalised. It's lowercase when there's a second letter.
Examination of 1 to 20 elements of the periodic table
-
Hydrogen
Under normal circumstances, hydrogen is a nonmetallic, colourless gas. It transforms into an alkali metal under great pressure. This element has three isotopes, each with a different amount of neutrons in its atoms. Protium is the most prevalent isotope. Deuterium and tritium are the other two.
-
The atomic number of Hydrogen is 1
-
H is its symbol
-
It is estimated to have an atomic mass value of 1.008 amu
-
H is assigned 1s1 configuration
-
It is a Nonmetal belonging to group 1 and s-block
2. Helium
-
Helium is a light gas with no visible colour.
-
The atomic number of Helium is 2
-
He is its symbol
-
It is estimated to have an atomic mass value of 4.002 amu
-
He is assigned 1s2 configuration
-
It is belonging to group 18 and s-block
3. Lithium
It is a highly reactive solid metal with a silver colour
-
The atomic number of Lithium is 3
-
Li is its symbol
-
It is estimated to have an atomic mass value of 6.94 amu
-
Li is assigned [He] 2s1 configuration
-
It is an alkali metal belonging to group 1 and s-block.
4. Beryllium
It is a solid material with a shiny grey- white appearance
-
The atomic number of beryllium is 4
-
Be is its symbol
-
It is estimated to have an atomic mass value of 9.012 amu
-
Be is assigned [He] 2s2 configuration
-
It is an alkaline earth metal belonging to group 2 and s-block.
5. Boron
-
The atomic number of Boron is 5
-
B is its symbol
-
It is estimated to have an atomic mass value of 10.81 amu
-
B is assigned [He] 2s2 2p1 configuration
-
It is a metalloid belonging to group 13 and p-block.
6. Carbon
-
The atomic number of carbon is 6
-
C is its symbol
-
It is estimated to have an atomic mass value of 12.011 amu
-
C is assigned [He] 2s2 2p2 configuration
-
It is a metalloid belonging to group 14 and p-block
7. Nitrogen
-
the atomic number of nitrogen is 7
-
N is its symbol
-
It is estimated to have an atomic mass value of 14.007 amu
-
N is assigned [He] 2s2 2p3 configuration
-
It belongs to group 15 and p-block.
8. Oxygen
-
The atomic number of oxygen is 8
-
O is its symbol
-
It is estimated to have an atomic mass value of 16 amu
-
O is assigned [He] 2s2 2p4 configuration
-
It is belonging to group 16 and p-block
9. Fluorine
-
The atomic number of fluorine is 9
-
F is its symbol
-
It is estimated to have an atomic mass value of 18.989 amu
-
F is assigned [He] 2s2 2p5 configuration
-
It is belonging to group 17 and p-block
10. Neon
-
the atomic number of neon is 10
-
Ne is its symbol
-
It is estimated to have an atomic mass value of 20.179 amu
-
N is assigned [He] 2s2 2p6 configuration
-
It is belonging to group 18 and p-block
11. Sodium
-
The atomic number of sodium is 11
-
Na is the symbol of sodium
-
The Latin name of sodium is Natrium
-
It is estimated to have an atomic mass value of 22.989 amu
-
F is assigned [Ne] 3s1 configuration
-
It is belonging to group 1 and s-block
12. Magnesium
-
The atomic number of magnesium is 12
-
Mg is its symbol
-
It is estimated to have an atomic mass value of 24.309 amu
-
Mg is assigned [Ne] 3s2 configuration
-
It is belonging to group 2 and s-block
13. Aluminium
-
The atomic number of aluminium is 13
-
Al is its symbol
-
It is estimated to have an atomic mass value of 26.968 amu
-
Al is assigned [Ne] 3s2 3p1 configuration
-
It is belonging to group 13 and p-block
14. Silicon
-
The atomic number of Silicon is 14
-
Si is its symbol
-
It is estimated to have an atomic mass value of 28.085 amu
-
Si is assigned [Ne] 3s2 3p2 configuration
-
It belongs to group 14 and p-block.
15. Phosphorous
-
The atomic number of Phosphorous is 15
-
P is its symbol
-
It is estimated to have an atomic mass value of 30.9737 amu
-
P is assigned [Ne] 3s2 3p3 configuration
-
It is belonging to group 15 and p-block
16. Sulphur
-
The atomic number of Sulfer is 16
-
S is its symbol
-
It is estimated to have an atomic mass value of 32.09 amu
-
S is assigned [Ne] 3s2 3p4 configuration
-
It is belonging to group 16 and p-block
17. Chlorine
-
The atomic number of Chlorine is 17.
-
Cl is its symbol
-
It is estimated to have an atomic mass value of 35.45 amu
-
Cl is assigned [Ne] 3s2 3p5 configuration
-
It is belonging to group 17 and p-block
18. Argon
-
the atomic number of argon is 18
-
Ar is its symbol
-
It is estimated to have an atomic mass value of 39.948 amu
-
Ar is assigned [Ne] 3s2 3p6 configuration
-
It is belonging to group 18 and p-block
19. Potassium
-
The atomic number of Potassium is 19
-
The Latin name of potassium is found to be Kalium
-
K is its symbol
-
It is estimated to have an atomic mass value of 39.093 amu
-
K is assigned [Ar] 4s1 configuration
-
It is belonging to group 1 and s-block
20. Calcium
-
The atomic number of Calcium is 20.
-
Ca is its symbol
-
It is estimated to have an atomic mass value of 40.10 amu
-
Ca is assigned [Ar] 4s2 configuration
-
It is belonging to group 14 and s-block
Also Read:
Recommended topic video
Some Solved Examples
Example.1 Choose the correct option:
1) (correct)The period of the element is determined by its highest shell
2)The period of the element is determined by its last orbital
3)The period of the element is determined by its valence shell electrons
4)The period of the element is determined by its valency
Solution
The period of the element is determined by its highest shell.
Hence, the answer is the option (1).
Example.2 Which pair of atomic numbers represents s-block elements
1)7,15
2)6,12
3)9,17
4) (correct)3,12
Solution
Out of the given elements, Z= 3 (Li) and Z= 12 (Mg) belong to the s Block of the periodic table.
Hence, the answer is the option (4).
Example.3 Newland’s octave law was successful in arranging:
1)Heavier elements
2) (correct)Lighter elements
3)Both
4)None
Solution
Newland’s octave law was successful in arranging lighter elements. After calcium, this law did not work accordingly.
Hence, the answer is the option (2).
Summary
The first 20 elements of the periodic table possess a range of chemical properties and their uses from simplest to most abundant elements like hydrogen and some essential elements of earth such as carbon, nitrogen and hydrogen these three elements are the main building blocks of many processes and also plays a vital role in the chemistry field and has various technological applications. Their characteristics are very diverse and interconnected. The first 20 elements of the periodic table contain metals such as sodium, magnesium, and aluminium. Metalliods such as silicon, and boron. Also, contains noble gases like neon and argon. Understanding all these elements helps us to appreciate the balance of nature and their application in chemistry.
Frequently Asked Questions (FAQs)
The first 20 elements of the periodic table are:
1. Hydrogen (H)
2. Helium (He)
3. Lithium (Li)
4. Beryllium (Be)
5. Boron (B)
6. Carbon (C)
7. Nitrogen (N)
8. Oxygen (O)
9. Fluorine (F)
10. Neon (Ne)
11. Sodium (Na)
12. Magnesium (Mg)
13. Aluminum (Al)
14. Silicon (Si)
15. Phosphorus (P)
16. Sulfur (S)
17. Chlorine (Cl)
18. Argon (Ar)
19. Potassium (K)
20. Calcium (Ca)
These elements represent a range of different properties and are fundamental to many aspects of chemistry.
Each chemical element has a symbol, which is usually derived from its English name or its Latin name. For example, hydrogen is represented by "H" and sodium by "Na," which comes from its Latin name "Natrium." Symbols are used to simplify chemical formulas, equations, and labels for easier communication in science. They help avoid confusion and allow scientists globally to share knowledge efficiently.
Yes, the first 20 elements can be categorized into several groups based on their properties. For example, the first two elements, hydrogen and helium, are gases at room temperature, while lithium, beryllium, sodium, and magnesium are metals. Additionally, carbon, nitrogen, oxygen, and sulfur are essential non-metals. The different categories reflect varying properties, such as reactivity and state of matter.
Learning about the first 20 elements is crucial because they form the basis of all matter. Understanding these elements helps students grasp fundamental concepts in chemistry, such as bonding, reactions, and the nature of compounds. Many of these elements are vital for life, such as carbon, nitrogen, and oxygen, which are essential components of biological molecules.
The properties of the first 20 elements greatly influence their applications in our daily lives. For instance, carbon is a key element in organic chemistry, forming the backbone of biological molecules like proteins and carbohydrates. Oxygen is essential for respiration, while metals like sodium and potassium play crucial roles in physiological functions in living organisms. Moreover, elements like silicon are used in technology, underpinning modern electronics. Understanding these properties can lead to better insights into their practical uses.
Also Read
13 Jun'25 11:36 PM
03 Jun'25 08:49 PM
06 Feb'25 11:45 PM
06 Feb'25 11:36 PM
16 Dec'24 11:23 PM
13 Nov'24 03:53 PM
23 Sep'24 01:11 PM

