Heat Energy - Definition, Sources Of Heat Energy And Examples, FAQs
Heat energy is a form of energy that plays an essential role in the natural processes around us. It flows from a higher temperature to a cooler temperature. In this article, we will be discussing what is heat energy, the definition of heat energy in science, sources of heat energy, heat energy examples, types of heat transfer, uses and applications of heat energy, and heat energy images.
JEE Main/NEET 2027: Physics Important Formulas for Class 10
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is Heat Energy?
- Transfer of Heat
- Conduction
- Convection
- Radiation
- Sources of Heat Energy
- Classification of Heat
- Uses of Heat Energy
- Heat Energy Examples
- Applications of Heat Energy

What is Heat Energy?
Heat energy definition: Heat energy is defined as energy flow from a warm substance to a cooler substance. Heat energy is also defined as thermal energy. Heat is the main form of energy for people to survive in this world. Heat energy is the main vital source for the earth. For example the change of seasons, the flow of wind happens because of this heat which prevails unevenly around the earth. The SI unit of heat energy is in joules (
The heat energy is everywhere around us. Even in volcanoes, icebergs, in every object heat is present. The movement of molecules, atoms, or ions particles in the solid, gas, and liquid is due to heat in the object. The heat will flow from one object to another object which depends upon the temperature difference. The heat will flow from warmer regions to colder regions.

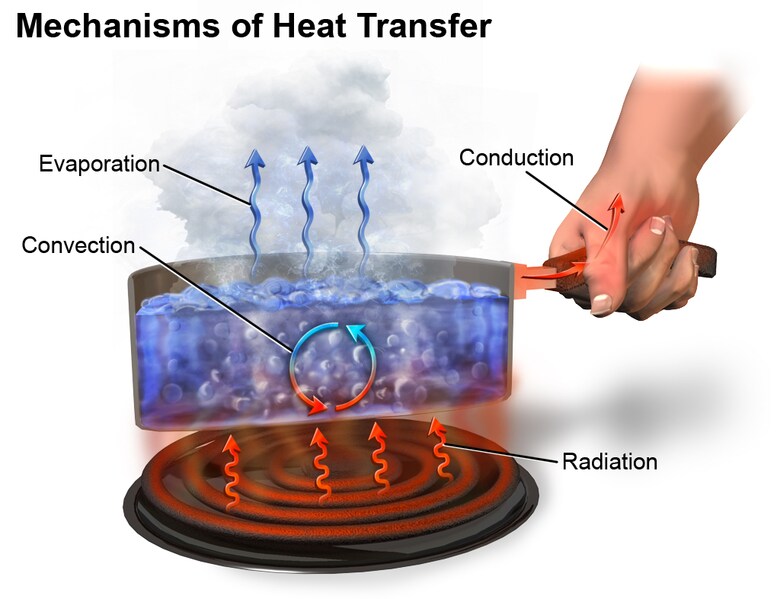
Transfer of Heat
When the temperature of the body is increased, the molecules in the body will start vibrating more. These vibrations will transfer from one part of the body to another part of the body. This vibrating energy of the molecules is termed as heat energy. The heat transfer from the heat region to the cold region. This will be termed as energy from the molecules that vibrate faster will transfer to the molecules that vibrate slower. This molecule's vibration is called heat content. When the heat content is high, then the body is hot.
Heat is a form of energy. Heat is the capacity to do work. For example, ice which is a solid material that absorbs heat will convert to liquid form like water. This process is called melting so here the heat is the energy which will convert to another form. Another example is boiling in which the liquid is converted to vapor phase. Heat is represented in Joules. Heat energy can be converted to another form like light energy using a bulb, or mechanical energy using a motor.
Heat energy will be transmitted in the following types
- Conduction
- Convection
- Radiation
Conduction
Conduction is the process of heat transfer that happens in a solid. The gases and liquids are poor conductors of heat. When the solid is heated, the molecules inside the solid will gain energy because of this the molecules will vibrate. During this process, the molecules when vibrated will transfer heat from one molecule to another molecule. Because of this process, the heat will transfer.
Convection
Heat transfer by convection is the process of heat transfer that happens in liquid and gas. When the water is heated up the water molecules will gain energy and move apart. The air molecules will become less dense and will rise. When it cooled down it became water again.
Radiation
Heat transfer by radiation is the process that occurs in the sun i.e. the transmission of heat energy from the sun. This type of transmission of heat energy does not require any medium. In this process, the heat waves from the hot object will radiate around it.
Sources of Heat Energy
- Sun, which is the main source of heat energy. Sun will be used as light energy, food resources, for electricity production.
- Electricity will produce heat energy through the stove, gas burners, and incandescent lamps.
- Chemical energy is used to produce heat energy through the chemical process.
- Nuclear energy is used to produce heat energy through nuclear processes like nuclear fusion.
So heat is the process of doing work. The heat is converted to various forms for some useful processes.
|
Related Topics, |
Classification of Heat
Heat is classified into
- Hot
- Cold
Hot is a term that refers to a body with high heat content. Examples of heat are the sun, stove, volcanic eruptions, etc.
Cold is a term that refers to a body with low heat content. Examples are air conditioners, cold water, etc.
Uses of Heat Energy
- For domestic purposes like cooking, agriculture needs
- For industrial purposes like electricity production, manufacturing purposes
- For production purposes like baking, and molding.
Heat Energy Examples
- The main supply of thermal energy is the sun
- A hot cup
- Stove burner
- Gas engines, automobile engines, diesel engines that heats up
- Hot gas balloon
Applications of Heat Energy
- Used for cooking
- Used in smelting lasses and glass production
- Welding and soldering
- Used in thermal imaging in the medical field
- Thermal batteries to store energy
- Greenhouses
- Used in renewable energy sources like biomass energy
Also read:
Frequently Asked Questions (FAQs)
Heat | Temperature |
Heat is referred to as transfer of energy in the system between the molecules. | Temperature is referred as the physical quantity which will outlines the coldness and hot of the object |
Heat is measured in Joules | Temperature is measured in Rankine and Celsius or Fahrenheit or Kelvin. |
Heat deals with heat energy | Temperature deals with kinetic energy of the molecules |
Principle of calorimeter is used to measure heat | Thermometer is used to measure temperature |
Heat energy depends upon mass, temperature, material of the object | Temperature depends upon the molecules kinetic energy |
The heat will flow from one object to another object which depends upon the temperature difference. i.e flows from hot object to cold object | Temperature of the body is high if the body is heated and low if the body is cools down |
The ability of doing work is heat | Temperature is measure of heat |
Heat is transmitted in three ways. They are
Conduction
Convection
Radiation
Conduction: Conduction is the process of heat transfer that happens in solid. The gases and liquid are poor conductors of heat.
Convection: Convection is the process of heat transfer that happens in liquid and gas.
Radiation: Radiation is the process of the transmission of heat energy from the sun. This type of transmission of heat energy does not require any medium.
The heat energy is everywhere around us. Even in volcanoes, icebergs, in every object heat is present. Heat is the main form of energy for the people to survive in this world. Heat energy is the main vital source for earth.
Sun
Electricity
Chemical energy
Nuclear energy
Domestic usage
Industrial function
Production function
The main supply of thermal energy is sun
A cup which is hot
Stove burner
Gas engines, automobile engines, diesel engines that heats up
Hot gas balloon
Yes, heat is a form of energy that can be transferred from one body to another.
Also Read
02 Jul'25 07:03 PM
02 Jul'25 05:33 PM
02 Jul'25 05:08 PM
02 Jul'25 05:07 PM
02 Jul'25 05:04 PM
02 Jul'25 05:04 PM
02 Jul'25 05:00 PM
02 Jul'25 05:00 PM
02 Jul'25 05:00 PM
02 Jul'25 04:58 PM

