Avogadros Number - Definition, FAQs
Avogadro's number or Avogadro constant is the relation factor which relates the number of constituent molecules, atoms or ions in a sample with the volume of a substance in that sample. In this article on Physics, we will answer the questions, what is Avogadro’s number or what is Avogadro’s constant? To study the Avogadro number in detail first, we have to learn about the term mole which we will study in this article.
JEE Main/NEET 2027: Physics Important Formulas for Class 10
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is a Mole?
- Ever wondered how many atoms are in the universe or in your body? How can you count a quantity as small as an atom?
- What is Avogadro's Number?
- Detailed Explanation of Avogadro's Number
- What Will be the 1 Mole of Pure Gold?
- What is Molar Volume?

What is a Mole?
A mole is defined as the amount of substance that contains the same number of atoms or molecules or particles as there are atoms in 12 grams of carbon-12.
1 mole of carbon-12 = 12 grams of carbon-12 = 6.022 ×10²³ atoms
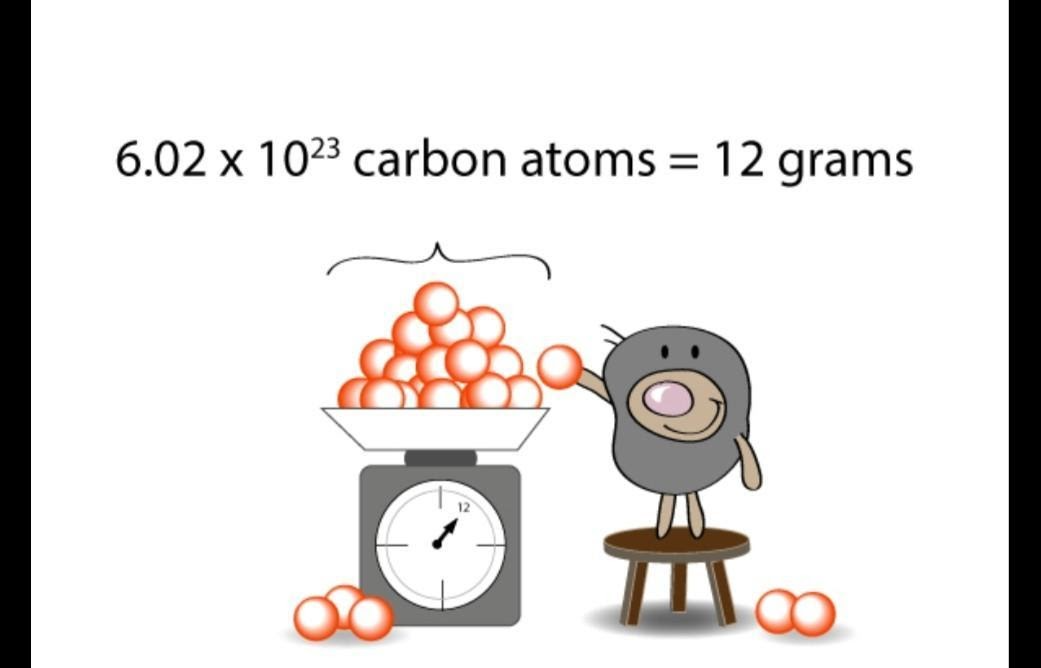 One mole of atoms = 6.022 ×10²³ atoms
One mole of atoms = 6.022 ×10²³ atoms
One mole of molecules = 6.022 ×10²³ molecules
In other words,
Number of particles in 1 mole = number of atoms in 1 mole of carbon-12
Where Carbon-12 is one of the isotopes of carbon and is used as a standard unit for Mole.
Ever wondered how many atoms are in the universe or in your body? How can you count a quantity as small as an atom?
To answer these questions, in 1811 Italian chemist Avogadro, the full name of Avogadro is Lorenzo Romano Amedeo Carlo Avogadro, was thinking of the atoms and molecules inside a flask from those ideas he came up with the thought of Avogadro's hypothesis which specified that "if the physical conditions are same that is temperature, pressure and volume then the molecules present will also be same”.

Let’s take an example to understand this statement further.
We take a container whose capacity is 100 ml and if we put hydrogen gas in it we measure the number of molecules and say the number of molecules comes out to be one lakh. Now, let’s take another container whose capacity is also 100 ml and put oxygen gas inside it. If the conditions are the same that is temperature, pressure and other physical quantities are the same. So, the number of molecules in oxygen gas will also be one lakh only under similar conditions of temperature and pressure.
But most of the scientists at that time looked at Avogadro's work as a hypothesis and didn't give it much thought to it. In 1860, his work was proven correct but unfortunately, Avogadro died in 1856. This hypothesis made it easier to perform calculations involving gases, chemists use it in the same way as we use grams to buy grapes, vegetables or meat. This means that 1 mole of any gas will occupy the same volume as 1 mole of any other gas provided that they are at the same temperature and pressure.
Also read -
What is Avogadro's Number?
The value of the Avogadro number is 6.022 ×10²³ also known as Avogadro's constant or NA. There is no difference between the Avogadro number and the Avogadro constant, they are one or the same thing. This is the same number of particles as there are in exactly 12 g of carbon-12. If we try to count Avogadro’s number and if 10000 people start to count Avogadro’s number and count at the rate of 100 numbers per minute each minute of the day, it would take over 1 trillion years to count the total number and if one mole of marbles were spread over the surface of the earth, our planet would be covered by a 20-mile dense layer of marbles.

Avogadro's number unit is mole inverse (mole-1)
|
Related Topics, |
Detailed Explanation of Avogadro's Number
Avogadro number is 6.022 ×10²³ just a number to represent quantities. It is similar to how we use the word 1 pair to represent 2, 1 dozen to represent 12, 1 baker’s dozen to represent 13, 1 gross to represent 144 and 1 Ream to represent 500. Similarly, the Avogadro number is the term to represent the 6.022 ×10²³ number of any entity. Avogadro's number is not used by us in daily life and is not advisable. So why do we use the number that exists if we do not use it in daily life? Yes, the number is not used in everyday life though it approaches the rescue of chemists who work with atoms, molecules, electrons and many more such small entities. That is the reason why it is used to calculate 1 mole of any substance.
1 mole of carbon-12 = 12 grams of carbon-12 = 6.022 ×10²³ atoms
What Will be the 1 Mole of Pure Gold?
1 mole of pure gold = 196 grams of pure gold = 6.022 ×10²³ atoms
Similarly,
1 mole of pure water = 18 grams of pure water = 6.022 ×10²³ molecules
But there is a point to be noted if we take one mole of gold, one mole of silver and one mole of copper.
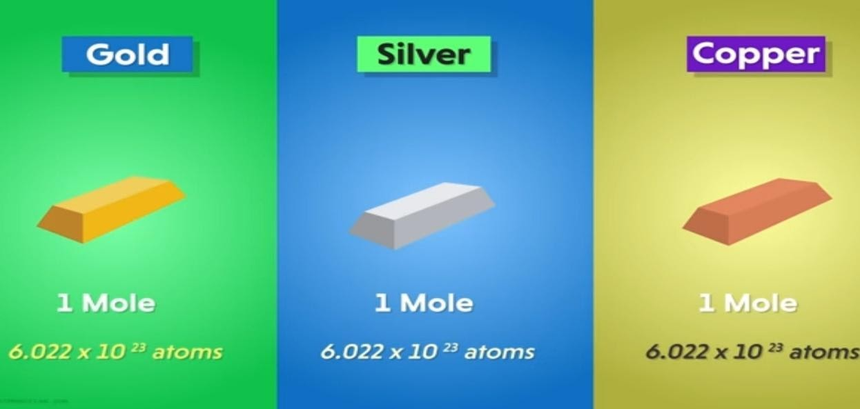
They will have the same number of atoms that is 6.022×10²³ atoms but the mass of each one will be different because the mass of individual atoms makes these elements different.
What is Molar Volume?
Molar volume is defined as the volume occupied by one mole of gas. Molar volume is measured in dm³mol¹.
At room temperature and pressure, the molar volume is taken as 24 dm³mole-1.
Let's solve an example to get it better.
Find the volume occupied by 8 grams of O2 at room temp.
1 mole = 24dm³
And,
1 mole of O2 = 32g
32 g of O2= 24 dm³
1g = 24dm³/32
So,
4g= 4×24 dm³/32
=3dm³
How Avogadro’s number (NA) is associated with other constants
Avogadro’s number is associated with other physical constants and properties in the following ways:
-
The relation between R which is the molar gas constant, NA which is Avogadro’s number and Kb which is Boltzmann’s constant is
R = Kb. NA
Where, Kb=1.38 X 10-23J/K and NA=6.022×10²³
=8.3144 J.K.mol
-
The relation between Faraday constant (F), Avogadro’s number (NA) and elementary charge (e) is
F = e.NA
Where, e= 1.6 x 10-19 C and NA= 6.022×10²³
=96485.3321 C/mol
-
The relation between the molar mass constant (Mu), Avogadro’s number (NA) and atomic mass constant (mu) is
Mu=mu.NA
Where, mu= 1.66 x10-27 Cmol and NA=6.022×10²³
=0.9999999 x10-3 kg. mol-1
A few questions related to Avogadro’s number example and moles
-
Find several moles of atoms in 120 g of calcium. Also, find the number of atoms present.{Ca =40}
Number of moles = mass given/molar mass = 120/40
=3 moles of calcium atoms
For the number of atoms present,
One mole = 6.022 ×10²³ atoms
=3 x 6.022 x 10²³ calcium atoms
-
Find several moles of molecules in 34 g of NH3. And the number of molecules present. [N=14,H=1]
Number of moles = mass given/molar mass = 34/17
= 2 moles of NH3 molecule
For the number of molecules present,
One mole = 6.022 ×10²³ molecules
=2x 6.022 x 10²³ NH3 molecules
-
Find the atoms which are present in 42.67 mole of copper.
One mole = 6.022 ×10²³ molecules
For the number of atoms present in 42.67 mole of copper,
=42.67 x 6.022 x 10²³ atoms
=2.570 x 1025 atoms
-
Find the molecules which are present in 7.32 moles of sulphur dioxide.
One mole = 6.022 ×10²³ molecules
For the number of molecules present in 7.32 mole of sulphur dioxide,
=7.32 x 6.022 x 10²³ molecules
=4.41 x 1024 molecules
Frequently Asked Questions (FAQs)
Full name of Avogadro - Lorenzo Romano Amedeo Carlo Avogadro
1 Mole is defined as the amount of substance that contains the same number of atoms or molecules or particles as there are atoms in 12 grams of carbon-12.
Yes, Avogadro number and Avogadro constant are same.
Unit of Avogadro is mole inverse.
In 1811, Avogadro number was discovered.
Also Read
28 Nov'24 12:03 PM
26 Nov'24 05:01 PM
17 Nov'24 02:39 PM
12 Nov'24 11:26 PM
12 Nov'24 11:25 PM
12 Nov'24 09:25 PM
12 Nov'24 06:03 PM
12 Nov'24 02:15 AM
12 Nov'24 01:39 AM
12 Nov'24 01:06 AM

