Some Basic Concepts of Chemistry - Notes, Topics, Formula, Books, FAQs
Some Basic Concepts of Chemistry" is the branch of chemistry that deals with the concept of fundamentals. It discusses the mole concept with Avogadro's number and Dalton's Atomic Theory. The chapter also describes the idea of stoichiometry, which is the quantitative relationships between reactants and products in a chemical reaction, and also gives the idea about computing empirical and molecular formulas. The limiting reagent, percentage composition, and other methods of expressing solution concentration, such as molarity and molality, are also taken into account. All things considered, this chapter offers the fundamental ideas and instruments required in dealing with chemistry problems.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Important Topics of Some Basic Concepts in Chemistry
- Overview of the Chapter
- Nature of Matter
- Laws of Chemical Combination
- Dalton's Atomic Theory
- Mole Concept
- Empirical Formula and Molecular formula
- Stoichiometry and Stoichiometric Calculations
- Some important Concentration Terms
- How do you prepare for some basic concepts of chemistry?
- Real-life application based on Some Basic Concepts in Chemistry
- Prescribed Books for some basic concepts of chemistry
- Some Solved Examples
- Some Important PYQs -
- Conclusion
Important Topics of Some Basic Concepts in Chemistry
Nature and Characteristics of Matter
Matter is anything that occupies space and has mass. Matter exists in three physical states—solid, liquid, and gas and can also be classified as pure substances or mixtures based on its composition. The study of nature and characteristics of matter forms the foundation of chemistry.
Properties of Matter and Their Measurement
Matter exhibits physical properties, like colour, density, and boiling point, and chemical properties, like reactivity and flammability. These properties are measured using SI units, ensuring consistency in scientific communication.
Uncertainty in Measurement
All measurements involve some degree of uncertainty due to limitations in instruments or human error. Significant figures and error analysis help quantify and minimize these uncertainties in measurement in scientific calculations.
Atomic Mass and Molecular Mass
Atomic mass is the weighted average mass of an element’s isotopes, while molecular mass is the sum of the atomic masses of all atoms in a molecule. Atomic mass and molecular mass are crucial for stoichiometric and chemical calculations.
Mole Concept
The mole is a fundamental unit in chemistry that represents 6.022×1023 (Avogadro's number). Mole concept is used to relate the mass of a substance to the number of particles it contains, enabling easy quantitative analysis.
Percent Composition Formula
Percent composition indicates the percentage by mass of each element in a compound. It helps in determining the compound's empirical and molecular formulas, providing insights into its composition.
Empirical and Molecular Formula
The empirical formula represents the simplest whole-number ratio of atoms in a compound, while the molecular formula shows the actual number of atoms of each element. Empirical and Molecular formulas are derived using percent composition and molecular mass.
Stoichiometric Calculations
Stoichiometry deals with quantitative relationships in chemical reactions. Stoichiometric calculations involve balancing chemical equations and determining the amounts of reactants or products, ensuring the conservation of mass and atoms.
Gravimetric Analysis
Gravimetric analysis is a quantitative technique where the mass of a substance is measured to determine its composition or concentration. It is highly accurate and commonly used in laboratories.
Law of Equivalence
The law of equivalence states that the amount of one substance reacting with or replacing another is proportional to its equivalent masses. This principle simplifies stoichiometric calculations.
Oleum and Its % Labelling
Oleum also known as fuming sulfuric acid is labelled based on the percentage of free SO3 it contains. This labeling aids in its safe handling and precise use in industrial applications.
Some Basic Concepts in Chemistry Formula
Some basic concepts in chemistry formulas are related to the mole formula, percent composition, and stoichiometry-related equations, which are integral to solving chemical problems.
Laws of Chemical Combination for Elements and Compounds
Laws of chemical combination for elements and compounds include the law of conservation of mass, the law of definite proportions, and the law of multiple proportions. These laws describe how elements combine to form compounds, emphasizing the fixed and predictable nature of chemical reactions.
Molarity and Mole Fraction
Molarity measures the concentration of a solution in moles per liter, while the mole fraction expresses the ratio of moles of a component to the total moles in the mixture. Molarity and mole fraction are important topics for solution chemistry and reaction kinetics.
Overview of the Chapter
Some basic concepts of chemistry are the most basic chapter of Chemistry. It has various important concepts that you need to have greater insights for a better understanding of the whole of chemistry. This article will help you to give important insights into this chapter and will also guide you in some important tips and guidelines.
Nature of Matter
Everything in the universe that has some mass and occupies some space is known as matter. Matter exists in three different physical forms i.e., solid, liquid, and gas.
| Property | Solid | Liquid | Gas |
| Tightness | Very tightly packed | Tightly packed | Loosely packed |
| Intermolecular space | Minimum | Intermediate | Maximum |
| Force of attraction | Maximum | Intermediate | Minimum |
| Kinetic Energy | Minimum | Intermediate | Maximum |
| Density | Maximum | Intermediate | Minimum |
| Volume | Fixed | Fixed | Variable |
| Shape | Fixed | Variable | Variable |
| Compressibility factor | Minimum | Intermediate | Maximum |
At the macroscopic level, the matter can be classified into two categories i.e, mixtures and pure compounds as shown in the figure.
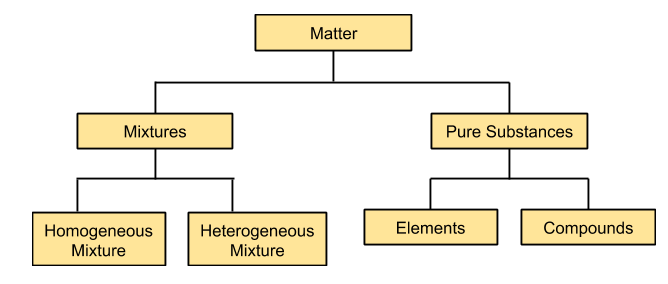
Mixtures are those substances in which two or more components are mixed. Mixtures are further classified as homogeneous and heterogeneous mixtures. Homogeneous mixtures are the ones in which components are present in a fixed ratio and the properties of this kind of mixture are the same throughout, for example, solution of sugar in water. However heterogeneous mixtures are those in which the components are not mixed in a definite ratio and properties of the mixture vary at different positions of the mixture, for example, sand in water.
Laws of Chemical Combination
The combination of elements to form some new product follows three basic laws as shown in the figure
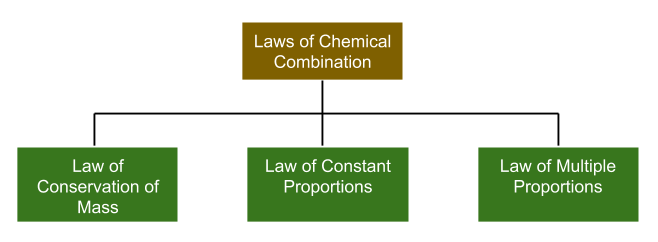
- Law of conservation of mass: This law states that "matter can neither be created nor destroyed". This simply means that the number of reactants that are used in the reaction will be equal to the number of products formed.
- Law of definite proportions: This law states that any compound always contains its components in the fixed ratio by mass irrespective of its source and method of preparation.
- Law of multiple proportions: This law states that when two elements combine to form one or more than one compound, then the masses of one element that combine with the other element are in the ratio of small whole numbers.
Dalton's Atomic Theory
John Dalton proposed a theory about matter and atoms in which he proposed the following postulates:
(i) Matter consists of small individual particles known as 'atoms'.
(ii) All atoms of any particular element have the same properties and same mass but atoms of different elements have different properties and mass.
(iii) Compounds are formed when atoms of different elements are combined in a fixed ratio.
(iv) Chemical reactions involve the reorganization of atoms.
Mole Concept
A mole is the standard unit to measure the number of particles like atoms or molecules in a given sample. Mathematically, one mole is equal to 6.022 x 1023. In other words, it can be said that it is equal to as many particles as there are atoms in 12g of the carbon-12 isotope. For example, if we have one mole of oxygen gas, that means we have 6.022 x 1023 molecules of O2.
Empirical Formula and Molecular formula
The empirical formula is the simplest whole-number ratio of atoms present in any particular molecule and the molecular formula is the actual representation of the number of atoms present in the molecule.
For example glucose i.e. C6H12O6, its molecular formula is C6H12O6 but its empirical formula is CH2O.
Mathematically, the relation between empirical formula mass and molecular formula mass is given as follows:
n= Molecular mass empirical formula mass
where n is the simplest ratio.
Limiting Reagent
The reactant is consumed first in the reaction. When we are dealing with the balanced chemical equation, if the number of moles of reactants is not in the ratio of the stoichiometric coefficient of the balanced chemical equation, then there should be one reactant that should be the limiting reactant.
Percentage yield
Sometimes, experimentally, the reaction does not undergo 100% completion because of many factors which are involved in the actual industrial processes. So in such cases, we need the concept of % yield.
It is defined as the ratio of actual moles of product(s) formed to the number of moles that should have been theoretically formed assuming 100% completion of the reaction.
Stoichiometry and Stoichiometric Calculations
This concept helps us to calculate the mass or amount of reactants and products in the given chemical reaction. For calculations, first, we must have a balanced chemical equation, only then can we predict the mass of reactants and products. For example
The chemical reaction is given as follows:2H2+O2→2H2O
Now, this chemical equation is a balanced equation, thus we are able to predict that one mole of oxygen will combine with 2 moles of hydrogen and form 2 moles of water, and accordingly, we can calculate the masses of the respective elements.
In this concept, we also study the "limiting reagent". It is the substance that is present in reactants in a smaller amount. In other words, a limiting reagent is a substance that will be completely finished or react in the given chemical reaction.
Some important Concentration Terms
- Mole fraction: It is the ratio of the moles of any substance present in the solution to the total moles of the solution. Mathematically, it is given as follows:
Mole fraction of A=nA+nB
where nA is the moles of component A and nB is the moles of component B. - Molarity: It is the concentration of any substance present in the solution. In other words, it is moles of solute per unit volume of solution in litres. Mathematically, it can be given as follows:
Molarity = Moles of solute Volume of solution in litre. - Molality: It is the moles of solute dissolved in the given amount of solvent. Mathematically, it can be represented as follows:
Molality = Moles of solute Mass of solvent (Kg) - Normality: It is defined as the number of equivalents of solute dissolved in a one-litre volume of solution. Mathematically, it can be represented as follows:
Normality = Number of equivalents of solute Volume of solution in litre.
How do you prepare for some basic concepts of chemistry?
- This chapter is the beginning of chemistry. This chapter is one of the most important chapters of the complete chemistry syllabus. Its concepts, laws, numerals, and graphs all are important both for the basic foundation of chemistry and for scoring good marks in the examination.
- Read this chapter carefully, as it has all the basic concepts like the mole concept, stoichiometry, molarity, normality, etc.
- This chapter is the foundation stone of the whole chemistry syllabus.
- The rest of this chapter is very simple, just be regular and be consistent in your numerical practice.
Real-life application based on Some Basic Concepts in Chemistry
- In cooking, it is always necessary to know the exact amount of ingredients to be added to the food to get the best and most tasty dish. This calculation of ingredients in stoichiometry.

- Saturn planet is much larger than Earth but still, it is less dense. The specific gravity of Saturn is even less than 1.

- The GPS system that we use for finding out the way is related to stoichiometry. All these signals come from satellites. The stoichiometry helps to calculate the fuel and its components of reactions in the complete journey of satellites.

Prescribed Books for some basic concepts of chemistry
First, you must finish the class XI NCERT textbook and solve each and every example and unsolved question given in it. Then, for advanced level preparation like JEE and NEET, you must follow R.C. Mukherjee and O.P. Tandon. You must definitely solve the previous year's papers. Meanwhile, in the preparation, you must continuously write mock tests to the depth of knowledge. Our platform will help you to provide a variety of questions for deeper knowledge with the help of videos, articles, and mock tests.
Recommended topic video on (Some Basic Concepts of Chemistry)
Some Solved Examples
Example 1:
A sample of KCl is placed in 50 ml of solvent. What should be the mass (in gm) of the sample for the molarity to be 2M ?
1) (correct) 7.45
2) 7.81
3) 6.81
4) 7
Solution
Number of Moles = molarity x volume
= 2 x 0.05 = 0.1
So, mass = (39 + 35.5) x 0.01g = 7.45 g
Hence, the answer is an option (1).
Example 2:
The amount (in g) of sugar (C12H22O11) required to prepare 2L of its 0.1 M aqueous solution is:
1) 17.1
2) (correct) 68.4
3) 136.8
4) 34.2
Solution
Molarity -Molarity (M) = (Number of moles of solute)/(volume of solution in litres)
It is defined as the number of moles of the solute in 1 litre of the solution.
As we have learned in the mole concept.
The formula of molarity =(n)solute Vsolution ( in lit )
0.1=wt3422
wt(C12H22O11) = 68.4 gram
Hence, the answer is the option (2).
Example 3:
The amount of calcium oxide produced on heating 150 kg limestone (
Given : Molar mass (in
Solution-
Hence, the answer is 63 kg.
Some Important PYQs -
Q. Choose the correct statements.
(A) Weight of a substance is the amount of matter present in it.
(B) Mass is the force exerted by gravity on an object.
(C) Volume is the amount of space occupied by a substance.
(D) Temperatures below
(E) Precision refers to the closeness of various measurements for the same quantity.
Answer-
(A) Incorrect – The weight of a substance is the force exerted on it due to gravity, not the amount of matter present. The mass is the amount of matter in a substance.
(B) Incorrect – Mass is the amount of matter in an object, whereas weight is the force exerted by gravity on an object.
(C) Correct – Volume is indeed the amount of space occupied by a substance.
(D) Correct – Temperatures below 0°C are possible in the Celsius scale (e.g., -10°C, -50°C, etc.), but in the Kelvin scale, temperatures cannot be negative because 0 K (absolute zero) is the lowest possible temperature.
(E) Correct – Precision refers to the closeness of multiple measurements for the same quantity, even if they are not necessarily close to the actual (true) value.
Statement (C), (D) and (E) are correct
Conclusion
Basic concepts of chemistry tell about various important concepts like the Mole concept and stoichiometry which play an important role in industry-based setups to find the required amount of reagents. Various chemical reactions and their limiting agents can be identified using these concepts. Students are advised to read the prescribed books above in this article to get a good command of the entire subject.
Also read,
Frequently Asked Questions (FAQs)
Significant figures are important because they convey the precision of measurements and calculations. Using the correct number of significant figures helps to avoid overestimating the accuracy of results.
Significant figures affect calculations by ensuring that the precision of measurements is maintained throughout calculations. When performing operations (addition, subtraction, multiplication, division), the result should be reported with the appropriate number of significant figures based on the least precise measurement involved.
The laws of chemical combination include:
- Law of Conservation of Mass: Matter is neither created nor destroyed in a chemical reaction.
- Law of Definite Proportions: A chemical compound always contains the same proportion of elements by mass.
Law of Multiple Proportions: When two elements form more than one compound, the ratios of the masses of one element that combine with a fixed mass of the other can be expressed as small whole numbers.
Uncertainty can be minimized by using precise instruments, following proper laboratory techniques, and taking multiple measurements to average results.
The laws of chemical combination are fundamental to stoichiometry as they govern how reactants combine and the ratios in which they do so, allowing chemists to predict the amounts of products formed in reactions.
Also Read
03 Jun'25 09:04 PM
03 Jun'25 08:35 PM
17 Dec'24 10:32 AM
09 Dec'24 10:47 AM
09 Dec'24 10:45 AM
18 Oct'24 11:04 AM
18 Oct'24 10:57 AM

