1. What is Unification of Force?
The unification of forces is that the concept it's possible to weave all of nature’s force into one comprehensive force. Scientists have made great strides toward understanding how the forces are often combined.
Newton realized that an equivalent gravity that describes an apple falling from the tree also describes the moon’s orbit round the Earth. Later within the 19th century, James Clerk Maxwell demonstrated that electric and magnetism to be a facet of one electromagnetic force. Finally, within the 20th century, Weinberg, Abdus Salam, and Sheldon Lee Glashow discovered that at high energies, the electromagnetic force and therefore the weak interaction merge into one electroweak force.
2. What are the Universal Forces?
The four fundamental forces, also referred to as the Universal forces are electromagnetic force, strong nuclear force, weak nuclear force, and gravitation. Among these forces, gravitation is that the weakest and therefore the strong nuclear force is that the strongest. The strong nuclear force acts over a little distance while gravity acts over an extended distance.
3. Which is that the strongest force in nature?
The strongest force in nature is that the strong nuclear force. it's also referred to as strong nuclear interaction and is that the strongest compared to the opposite forces that exist in nature. The forces are:
(a) Gravity
(b) The weak interaction (Weak nuclear interaction)
(c) Electromagnetism
(d) The strong interaction (Strong nuclear interaction)
4. Name the factors affecting the worth of acceleration thanks to gravity.
Following are the factors affecting the worth of acceleration thanks to gravity:
Height from the earth’s surface
Latitude of the plane
Depth
5. What is Ampere law?
Ampere law is additionally referred to as Ampere circuital law which states that the closed integral of the magnetic flux intensity is that the same because the current enclosed by it.
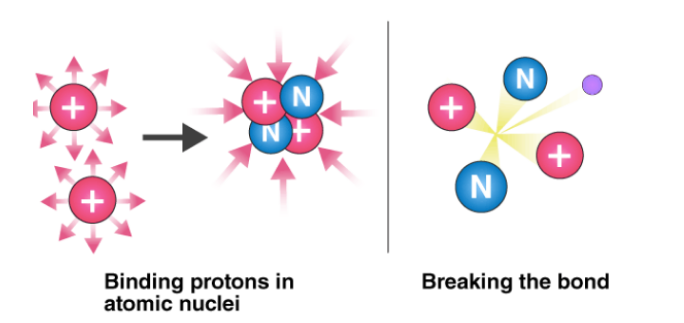
6. Why doesn't the nuclear force cause protons to collapse into each other?
The nuclear force has a property called "saturation," which means it only acts between nearest neighbors in a nucleus. This prevents all protons from attracting each other simultaneously and collapsing into a single point.
7. What is the range of the nuclear force?
The nuclear force has an extremely short range of about 1 femtometer (10^-15 meters). Beyond this distance, its strength drops off rapidly to essentially zero.
8. How does the nuclear force differ from electromagnetic force?
The nuclear force is much stronger than the electromagnetic force at short distances, but it has a much shorter range. While electromagnetic force decreases with distance according to an inverse square law, the nuclear force remains roughly constant up to about 1 femtometer and then drops off rapidly to zero.
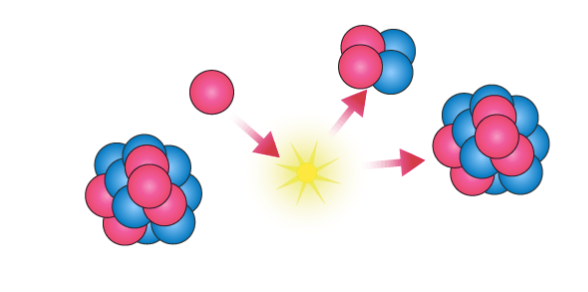
9. How does the nuclear force overcome electrostatic repulsion between protons?
At very short distances (less than 1 femtometer), the nuclear force is much stronger than the electromagnetic repulsion between protons. This allows it to overcome the electrostatic repulsion and bind protons together in the nucleus.
10. What particles mediate the nuclear force?
The nuclear force is mediated by particles called gluons, which are exchanged between quarks (the subatomic particles that make up protons and neutrons).
11. What is the nuclear force?
The nuclear force, also known as the strong nuclear force, is the fundamental force that holds protons and neutrons together within atomic nuclei. It is the strongest of the four fundamental forces in nature, but acts only over extremely short distances (about 1 femtometer, or 10^-15 meters).
12. What role does the nuclear force play in nuclear fission?
In nuclear fission, the nuclear force that holds a heavy nucleus together is overcome when the nucleus splits. This occurs when the electrostatic repulsion between protons in a large nucleus becomes greater than the nuclear force holding it together.
13. How does the nuclear force contribute to nuclear fusion?
In nuclear fusion, the nuclear force pulls light nuclei together when they come extremely close (within 1 femtometer). This overcomes the initial electrostatic repulsion between the positively charged nuclei, allowing them to fuse into a heavier nucleus.
14. What is the relationship between the nuclear force and binding energy?
The nuclear force is responsible for the binding energy of nuclei. The stronger the nuclear force between nucleons, the greater the binding energy, which is the energy required to break apart a nucleus into its constituent protons and neutrons.
15. How does the nuclear force affect nuclear stability?
The nuclear force plays a crucial role in nuclear stability. Stable nuclei have a balance between the attractive nuclear force and the repulsive electromagnetic force between protons. When this balance is disrupted, nuclei become unstable and may undergo radioactive decay.
16. How does the nuclear force affect the size of atomic nuclei?
The nuclear force determines the size of atomic nuclei by balancing against the electromagnetic repulsion between protons. As the number of nucleons increases, the volume of the nucleus grows approximately proportionally to the number of nucleons, a phenomenon known as the "liquid drop model."
17. How does the nuclear force affect nuclear decay processes?
The nuclear force influences nuclear decay processes by determining the stability of nuclei. When the balance between the nuclear force and electromagnetic repulsion is disrupted (e.g., in very heavy nuclei), it can lead to various forms of radioactive decay.
18. What is the relationship between the nuclear force and the semi-empirical mass formula?
The semi-empirical mass formula, which predicts nuclear binding energies, includes terms that represent the effects of the nuclear force. These include the volume term (representing the attractive nature of the force) and the surface term (accounting for nucleons at the surface having fewer neighbors to interact with).
19. What is the role of the nuclear force in neutron stars?
In neutron stars, the nuclear force plays a crucial role in counteracting the enormous gravitational pressure. It prevents the complete collapse of the star by providing a repulsive force at extremely high densities, supporting the star against further gravitational compression.
20. How does the nuclear force relate to the concept of "nuclear pasta" in astrophysics?
"Nuclear pasta" refers to exotic phases of matter thought to exist in neutron star crusts. The competition between the short-range nuclear force and long-range electromagnetic force at high densities can lead to unusual, pasta-like shapes of nuclear matter.
21. How does the nuclear force affect nuclear reactions?
The nuclear force governs nuclear reactions by determining the probability of nuclei coming close enough to interact. It provides the attractive force necessary to overcome the initial electrostatic repulsion between nuclei, allowing processes like fusion and fission to occur.
22. How does the nuclear force contribute to nuclear magnetic moments?
While the nuclear force doesn't directly cause magnetic moments, it influences their values by determining the spatial distribution and motion of protons and neutrons within the nucleus. This distribution affects the overall magnetic properties of the nucleus.
23. What is the relationship between the nuclear force and nuclear isomers?
Nuclear isomers are excited states of atomic nuclei that are relatively long-lived. The nuclear force plays a role in creating the energy levels that these isomers occupy and in determining the stability and decay properties of these excited states.
24. How does the nuclear force relate to the concept of "island of stability" for superheavy elements?
The "island of stability" refers to a predicted region of relatively stable superheavy elements. The nuclear force, through its interplay with quantum mechanical shell effects, is thought to provide extra stability to certain combinations of protons and neutrons in this region.
25. What is the role of the nuclear force in nuclear fission reactors?
In nuclear fission reactors, the nuclear force holds fissile nuclei together until they absorb a neutron. The resulting excited nucleus then overcomes the nuclear force binding it together, leading to fission. The products of this fission are also held together by the nuclear force.
26. How does the nuclear force contribute to the process of beta decay?
While beta decay is primarily governed by the weak nuclear force, the strong nuclear force (or nuclear force) plays an indirect role. It determines the nuclear structure and energy levels, which in turn affect the energetics and probability of beta decay processes.
27. What is the role of the nuclear force in neutron stars' "glitches"?
Neutron star glitches are sudden increases in rotation rate. The nuclear force plays a crucial role in the internal structure of neutron stars, including the formation of a superfluid neutron component. The interaction between this superfluid and the star's crust, both governed by the nuclear force, is thought to cause these glitches.
28. What is the significance of the nuclear force in explaining the existence of neutron halos in some nuclei?
Neutron halos are diffuse clouds of neutrons surrounding some very neutron-rich nuclei. The nuclear force, through its short-range nature and saturation property, allows these extra neutrons to form a halo structure instead of being tightly bound to the core.
29. How does the nuclear force relate to the concept of "neutron skin" in heavy nuclei?
A neutron skin refers to an excess of neutrons at the surface of heavy, neutron-rich nuclei. This phenomenon arises from the interplay between the short-range nuclear force and the long-range Coulomb repulsion, which pushes protons towards the center of the nucleus.
30. How does the nuclear force contribute to nuclear quadrupole moments?
Nuclear quadrupole moments arise from non-spherical charge distributions in nuclei. The nuclear force, by determining the spatial arrangement of protons and neutrons, directly influences these charge distributions and thus the resulting quadrupole moments.
31. Is the nuclear force always attractive?
The nuclear force is predominantly attractive between nucleons (protons and neutrons) at distances of about 1 femtometer. However, at extremely short distances (less than 0.7 femtometers), it becomes repulsive, preventing nuclei from collapsing.
32. How does the strength of the nuclear force compare to other fundamental forces?
The nuclear force is the strongest of the four fundamental forces in nature. It is about 100 times stronger than the electromagnetic force, about 10^13 times stronger than the weak nuclear force, and about 10^38 times stronger than gravity.
33. Why is the nuclear force not noticeable in everyday life?
Due to its extremely short range (about 1 femtometer), the nuclear force is only significant at subatomic scales. It doesn't affect macroscopic objects or interactions we observe in daily life.
34. What is meant by the "charge independence" of the nuclear force?
Charge independence means that the nuclear force acts equally between proton-proton, neutron-neutron, and proton-neutron pairs. This property helps explain why protons and neutrons behave similarly within nuclei despite their different charges.
35. How does the nuclear force relate to the concept of "magic numbers" in nuclear physics?
Magic numbers in nuclear physics refer to specific numbers of protons or neutrons that result in exceptionally stable nuclei. These numbers (2, 8, 20, 28, 50, 82, and 126) correspond to filled energy levels or shells, which are a consequence of how nucleons interact via the nuclear force.
36. How does the nuclear force relate to the concept of "magic numbers" in nuclear physics?
Magic numbers in nuclear physics (2, 8, 20, 28, 50, 82, 126) correspond to particularly stable nuclear configurations. While these numbers arise from quantum mechanical shell effects, the underlying interaction governing these shells is the nuclear force.
37. What is the connection between the nuclear force and quantum chromodynamics (QCD)?
The nuclear force is a residual effect of the strong interaction described by quantum chromodynamics (QCD). While QCD describes the interactions between quarks and gluons, the nuclear force emerges as the effective force between nucleons, which are composite particles made of quarks.
38. What is meant by the "exchange force" nature of the nuclear force?
The nuclear force is often described as an exchange force because it can be modeled as the exchange of virtual particles (pions or gluons) between nucleons. This exchange process creates the attractive force that binds nucleons together.
39. How does the nuclear force contribute to the mass defect of nuclei?
The nuclear force is responsible for the binding energy of nuclei, which manifests as a mass defect. When nucleons bind together, some of their mass is converted to binding energy (according to E=mc^2), resulting in the nucleus having slightly less mass than the sum of its constituent nucleons.
40. What is the significance of the nuclear force in stellar nucleosynthesis?
The nuclear force is crucial for stellar nucleosynthesis, the process by which stars create heavier elements. It enables fusion reactions in stellar cores, allowing lighter elements to combine into heavier ones and releasing energy that powers stars.
41. How does the nuclear force contribute to the "valley of stability" in the chart of nuclides?
The nuclear force, along with the electromagnetic force, determines the stability of nuclei. The "valley of stability" represents the band of stable nuclides where these forces are optimally balanced. Nuclei outside this valley are unstable and undergo radioactive decay.
42. What is the significance of the nuclear force in the formation of deuterium?
The nuclear force is essential for the formation of deuterium (an isotope of hydrogen with one proton and one neutron). It overcomes the electrostatic repulsion between the proton and neutron, allowing them to bind together to form the deuterium nucleus.
43. How does the nuclear force affect the binding energy curve?
The nuclear force is primarily responsible for the shape of the binding energy curve. The curve's peak around iron-56 reflects the optimal balance between the attractive nuclear force and the repulsive Coulomb force, explaining why both fusion of light nuclei and fission of heavy nuclei can release energy.
44. What is meant by the "saturation" property of the nuclear force?
Saturation refers to the property of the nuclear force where each nucleon interacts strongly only with its nearest neighbors. This explains why the density of atomic nuclei is roughly constant regardless of their size, and why the binding energy per nucleon plateaus for medium and heavy nuclei.
45. How does the nuclear force contribute to the "liquid drop model" of the nucleus?
The nuclear force's short range and saturation property are key to the liquid drop model. They explain why nuclei behave similarly to incompressible liquid drops, with a constant density and surface tension, properties that stem from the characteristics of the nuclear force.
46. What is the connection between the nuclear force and the shell model of the nucleus?
While the nuclear force is fundamentally responsible for binding nucleons, the shell model describes how nucleons arrange themselves in energy levels within the nucleus. The specific arrangements (shells) arise from the interplay of the nuclear force with the Pauli exclusion principle.
47. What is the role of the nuclear force in alpha decay?
In alpha decay, the nuclear force plays a dual role. It's responsible for binding the alpha particle (two protons and two neutrons) within the parent nucleus, but it also allows the alpha particle to tunnel through the potential barrier and escape from the nucleus.
48. How does the nuclear force relate to the concept of "nuclear drip lines"?
Nuclear drip lines represent the limits of nuclear stability, beyond which nuclei cannot hold additional neutrons or protons. The nuclear force determines these limits by balancing against the Coulomb repulsion and the quantum mechanical effects that govern nucleon binding.
49. What is the significance of the nuclear force in the production of elements heavier than iron in supernovae?
In supernovae, the extreme conditions allow the nuclear force to overcome the electrostatic repulsion between heavy nuclei, enabling rapid neutron capture (r-process). This process, driven by the nuclear force, is responsible for the creation of elements heavier than iron in the universe.
50. How does the nuclear force affect the process of neutron capture?
The nuclear force is crucial in neutron capture processes. It provides the attractive interaction that allows a nucleus to absorb an incoming neutron. The strength of this interaction influences the probability of capture and the resulting nuclear structure.
51. What is the significance of the nuclear force in explaining the abundance of elements in the universe?
The nuclear force is fundamental to nucleosynthesis processes that determine elemental abundances. It governs fusion reactions in stars, supernovae explosions, and neutron star mergers, all of which contribute to the creation and distribution of elements throughout the universe.
52. What is the significance of the nuclear force in explaining the "iron peak" in cosmic abundance?
The "iron peak" in cosmic abundance refers to the relatively high abundance of elements around iron. This peak is a direct consequence of the nuclear force, which makes nuclei around iron the most tightly bound, and thus the end products of stellar nucleosynthesis in massive stars.
53. How does the nuclear force contribute to the process of nuclear transmutation?
Nuclear transmutation involves changing one element into another through nuclear reactions. The nuclear force governs these reactions, determining the probability of different transmutation pathways and the stability of the resulting nuclei.
54. What is the role of the nuclear force in the formation of hypernuclei?
Hypernuclei are nuclei that contain one or more hyperons (particles containing strange quarks) in addition to protons and neutrons. The nuclear force, through its fundamental quark-gluon interactions, allows these exotic particles to be bound within the nuclear structure.
55. What is the significance of the nuclear force in explaining the "island of inversion" in nuclear physics?
The "island of inversion" refers to a region in the nuclear chart where the expected shell structure is altered. This phenomenon is a result of the complex interplay between the nuclear force and nuclear structure, leading to unexpected changes in the ordering of energy levels in certain neutron-rich nuclei.