1. What is the difference between fission and fusion?
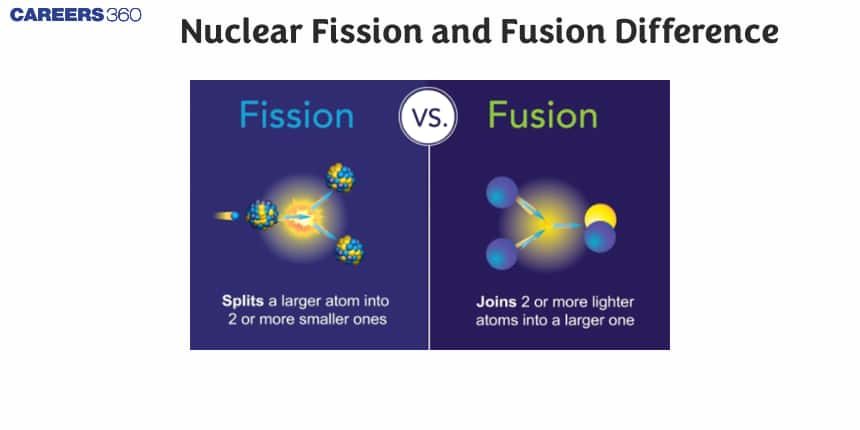
Both Nuclear fission and fusion are used for the production of a tremendous amount of energy but there are some differences between them too. The division of a single nucleus into smaller nuclei is nuclear fission while the combining of two or more small nuclei to form a heavier one is called nuclear fission. A nuclear fission reaction to initiate small energy is required for a nuclear fusion reaction; a large amount of energy is required to combine these small nuclei for the formation of a heavier one. The amount of energy released during a nuclear fusion reaction is very much higher than that of a nuclear fission reaction.
2. Distinguish between nuclear fission and nuclear fusion?
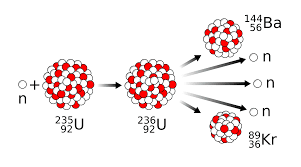
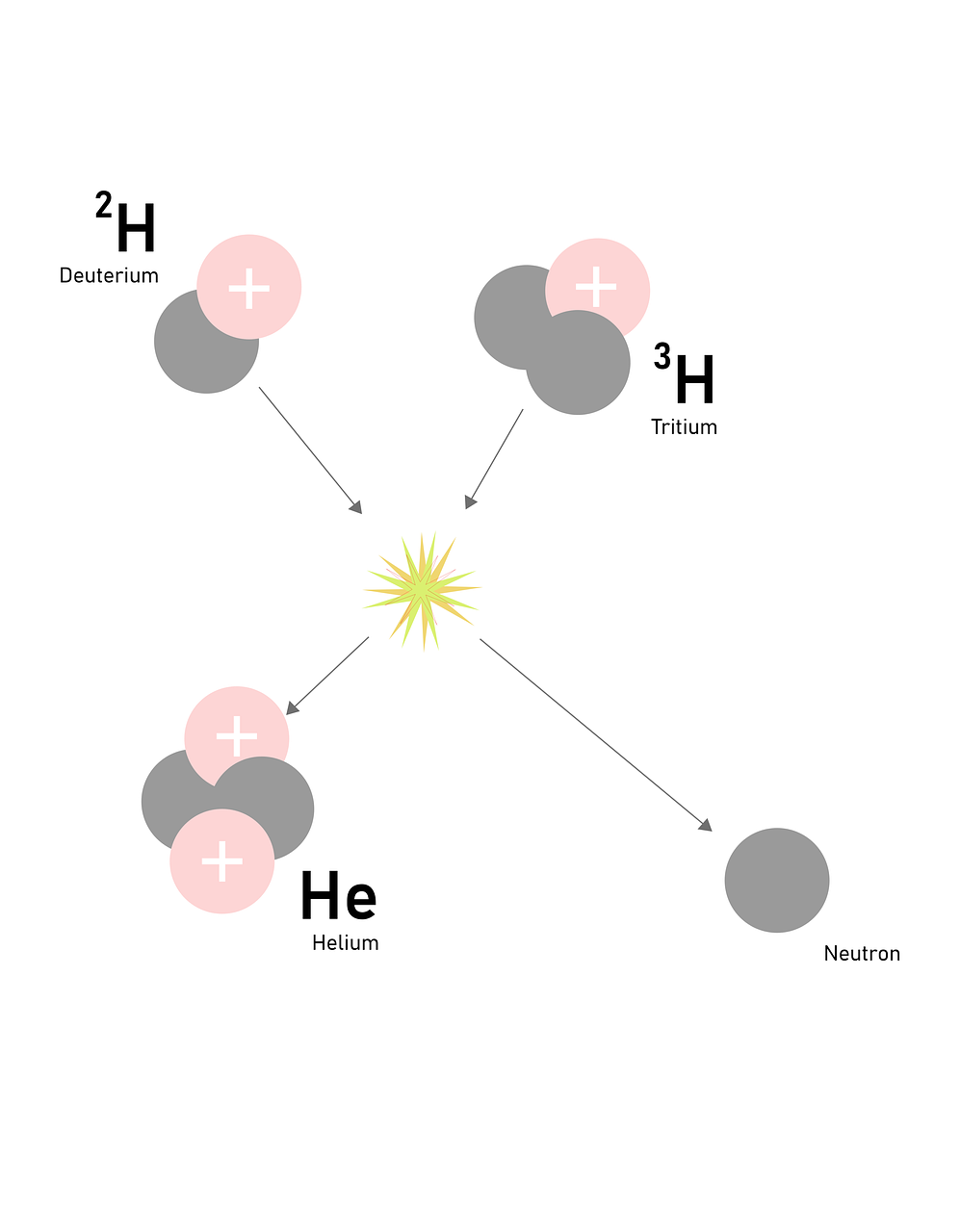
The reactions that are used for the production of a tremendous amount of energy is called nuclear fission and fusion reaction. Fission and fusion reactions are different since for a fission reaction to proceed it requires a very small amount of energy and with that energy, heavier nuclei are split into lighter nuclei with the release of energy. A very common example of nuclear fission is the splitting of Uranium-235 for the formation of barium. While a nuclear fusion reaction is a reaction where the required a large amount of energy to fuse the small nuclei and thereby forming a large nucleus with a tremendous release of energy. A very common example of a nuclear fusion reaction is the combination of deuterium and tritium for the formation of helium with the mass number 4.
3. Write any two difference between nuclear fusion and nuclear fission?
Both nuclear fission and fusion involve energy release but there is some difference too.
For fission, it requires small energy while fusion requires a large amount of energy.
The energy released after fusion is higher than that of a fission reaction.
4. Define nuclear fission and fusion for example.
Nuclear fission means the splitting of atomic nuclei into smaller units whereas nuclear fusion is the binding of nuclei together.
Example for nuclear fission: Uranium atom splits into xenon, iodine, barium, etc.
Example for nuclear fusion: A Hydrogen nucleus fuses to form helium.
5. Nuclear fission can be explained by which model?
Nuclear fission is explained by the liquid drop model.
6. What is the fundamental difference between nuclear fission and fusion?
Nuclear fission involves splitting heavy atomic nuclei into lighter ones, while nuclear fusion combines light atomic nuclei to form heavier ones. Both processes release energy, but fusion releases significantly more energy per unit mass than fission.
7. Why does fusion require extremely high temperatures to occur?
Fusion requires overcoming the electrostatic repulsion between positively charged nuclei. Extremely high temperatures (millions of degrees Celsius) provide the kinetic energy needed for nuclei to overcome this repulsion and get close enough for the strong nuclear force to take effect, allowing fusion to occur.
8. How does the binding energy per nucleon affect the energy release in fission and fusion reactions?
The binding energy per nucleon is highest for elements around iron (Fe-56). Fission of heavy nuclei and fusion of light nuclei both move towards this peak, releasing energy. Fission releases energy by breaking apart nuclei with lower binding energy per nucleon, while fusion combines nuclei with even lower binding energy per nucleon, both resulting in products closer to iron's stability.
9. Why is fusion considered a "cleaner" energy source than fission?
Fusion is considered cleaner because it produces no long-lived radioactive waste. The primary fusion reaction between deuterium and tritium produces helium, which is not radioactive. In contrast, fission produces various radioactive isotopes with long half-lives, requiring careful long-term storage and management.
10. What is the "island of stability" in nuclear physics, and how does it relate to fission?
The "island of stability" refers to a theoretical cluster of superheavy elements with relatively long half-lives due to their nuclear structure. It relates to fission because these elements, if they exist, would be less prone to spontaneous fission than other superheavy elements, potentially allowing for their study and use in nuclear research.
11. How does the neutron-to-proton ratio affect the stability of nuclei in fission reactions?
In fission reactions, the neutron-to-proton ratio is crucial for nuclear stability. Heavy elements have a higher neutron-to-proton ratio. When they undergo fission, the resulting smaller nuclei often have too many neutrons for their size, making them unstable. This leads to further radioactive decay of the fission products.
12. How does the mass defect relate to energy release in nuclear reactions?
The mass defect is the difference between the mass of a nucleus and the sum of its constituent nucleons' masses. This mass difference, according to Einstein's E=mc^2, is converted to energy during nuclear reactions. In both fission and fusion, the total mass of the products is slightly less than the reactants, with this mass difference being released as energy.
13. How do chain reactions differ between fission and fusion processes?
In fission, chain reactions occur when neutrons released from one fission event trigger subsequent fissions, potentially leading to an uncontrolled reaction if not properly managed. Fusion doesn't have a comparable chain reaction mechanism. Each fusion event is independent, requiring continuous input of energy to maintain the extreme conditions necessary for fusion to occur.
14. What is nuclear transmutation, and how does it relate to both fission and fusion?
Nuclear transmutation is the conversion of one element or isotope into another. In fission, it occurs when heavy nuclei split into lighter elements. In fusion, light elements combine to form heavier ones. Both processes result in the transmutation of elements. This concept is important in understanding the products of nuclear reactions and their potential applications or hazards.
15. How does the concept of half-life apply differently to fission products versus fusion products?
Half-life is crucial in fission reactions because many fission products are radioactive isotopes with varying half-lives, some very long. This creates challenges for waste management. In typical fusion reactions (like D-T fusion), the primary product is helium, which is stable. However, some fusion reactions can produce radioactive products, but generally with shorter half-lives compared to fission products.
16. Why is deuterium-tritium fusion considered the most promising for future fusion reactors?
Deuterium-tritium (D-T) fusion is considered most promising because it has the highest reaction rate at the lowest temperatures compared to other fusion reactions. This makes it easier to achieve and sustain in a reactor. Additionally, deuterium is abundant in seawater, and tritium can be bred from lithium within the reactor.
17. How does the concept of critical mass apply differently to fission and fusion reactions?
Critical mass is crucial for fission reactions, representing the minimum amount of fissile material needed to sustain a chain reaction. In fusion, there's no direct equivalent to critical mass. Instead, fusion requires achieving sufficient temperature, density, and confinement time (Lawson criterion) to sustain the reaction.
18. What role do moderators play in nuclear fission reactors, and why aren't they needed in fusion reactors?
In fission reactors, moderators slow down fast neutrons to thermal energies, increasing the probability of fission in fuel like U-235. Fusion reactors don't need moderators because the reaction doesn't rely on neutron-induced processes. Instead, fusion requires extremely high temperatures to overcome electrostatic repulsion between nuclei.
19. Why is hydrogen fusion the primary source of energy in stars, but not in current nuclear reactors on Earth?
Stars can sustain hydrogen fusion due to their enormous mass, which creates immense gravitational pressure and temperature at their cores. On Earth, we lack the ability to recreate these extreme conditions sustainably. Current nuclear reactors use fission because it's easier to control and doesn't require the extreme conditions necessary for fusion.
20. How does the concept of nuclear binding energy explain why both fission and fusion can release energy?
Nuclear binding energy is the energy required to break a nucleus into its constituent protons and neutrons. Both fission of heavy nuclei and fusion of light nuclei result in products with higher binding energy per nucleon. This increase in binding energy per nucleon is released as kinetic energy and radiation, explaining why both processes can produce energy.
21. How does the concept of nuclear pasta states relate to neutron stars and extreme nuclear physics?
Nuclear pasta states are theorized exotic phases of matter in neutron star crusts, where nuclear forces and electrostatic repulsion compete to create complex structures. While not directly related to terrestrial fission or fusion, understanding these states provides insights into nuclear physics under extreme conditions, potentially informing our understanding of nuclear reactions and matter at high densities.
22. How does the strong nuclear force overcome electrostatic repulsion in fusion reactions?
The strong nuclear force is extremely powerful but only acts over very short distances (about 10^-15 meters). In fusion reactions, nuclei must be brought close enough for the strong force to overcome the electrostatic repulsion. This is achieved through high temperatures that give particles enough kinetic energy to get sufficiently close despite the repulsion.
23. What is the significance of the "breakeven point" in fusion research?
The breakeven point in fusion research is when the energy produced by the fusion reaction equals the energy input required to initiate and sustain the reaction. Achieving breakeven is a crucial milestone in fusion research, as it demonstrates the potential for fusion as a net energy source. Surpassing breakeven is necessary for practical fusion power plants.
24. Why is controlled fusion more challenging to achieve than controlled fission?
Controlled fusion is more challenging because it requires extremely high temperatures (millions of degrees Celsius) and plasma confinement. These conditions are difficult to create and maintain on Earth. Fission, on the other hand, occurs at much lower temperatures and can be controlled using neutron-absorbing materials, making it easier to manage in a reactor setting.
25. Why is the sun able to fuse hydrogen into helium, but fusing helium into heavier elements requires more massive stars?
The sun fuses hydrogen into helium at its core temperature of about 15 million Kelvin. Fusing helium into heavier elements requires much higher temperatures (over 100 million Kelvin) due to the increased electrostatic repulsion between helium nuclei. Only more massive stars achieve these higher core temperatures, allowing them to fuse helium and heavier elements.
26. How does the concept of binding energy curve explain why iron-56 is the "peak" of nuclear stability?
The binding energy curve shows the average binding energy per nucleon for different elements. It peaks at iron-56, meaning iron has the highest binding energy per nucleon. Elements lighter than iron release energy through fusion, while those heavier release energy through fission, both processes moving towards the stability of iron. This explains why iron-56 is considered the most stable nucleus.
27. What is the role of neutron absorption in fission reactions, and why isn't it significant in fusion?
In fission reactions, neutron absorption by fissile nuclei (like U-235) is crucial for sustaining the chain reaction. Absorbed neutrons cause the nucleus to split, releasing more neutrons. In fusion, neutron absorption isn't significant because the reaction doesn't rely on neutron-induced processes. Instead, fusion depends on overcoming the electrostatic repulsion between nuclei.
28. How does quantum tunneling contribute to fusion reactions in stars?
Quantum tunneling allows particles to overcome energy barriers that classical physics would forbid. In stellar fusion, it enables protons to occasionally overcome the electrostatic repulsion and get close enough for the strong nuclear force to take effect, even at temperatures where classical physics suggests it shouldn't be possible. This makes fusion in stars more efficient than classical calculations would predict.
29. Why is the neutron-proton ratio important in determining the stability of heavy nuclei in fission reactions?
The neutron-proton ratio is crucial for the stability of heavy nuclei. As nuclei get heavier, they require more neutrons relative to protons to remain stable. This is because neutrons contribute to the strong nuclear force without adding to electrostatic repulsion. In fission, the resulting smaller nuclei often have too high a neutron-proton ratio, leading to their instability and subsequent decay.
30. How does the concept of nuclear shell model relate to the stability of certain isotopes in fission and fusion reactions?
The nuclear shell model, analogous to electron shells in atoms, describes energy levels in nuclei. "Magic numbers" of protons or neutrons (2, 8, 20, 28, 50, 82, 126) correspond to filled shells and increased stability. This affects fission by influencing which fragments are more likely to form, and in fusion, it helps explain why certain isotopes are more stable or easier to fuse than others.
31. What is the significance of the lawson criterion in fusion research?
The Lawson criterion defines the conditions necessary for a fusion reactor to reach ignition, where the heating of the fusion reactions is sufficient to maintain the temperature without external input. It states that the product of plasma density, confinement time, and temperature must exceed a certain value. Meeting this criterion is crucial for achieving sustainable fusion power generation.
32. How does the concept of cross-section apply differently to fission and fusion reactions?
Cross-section in nuclear physics represents the probability of a nuclear reaction occurring. In fission, it's important for determining the likelihood of a neutron causing a fission event, which varies with neutron energy. In fusion, cross-section is crucial for determining the probability of nuclei fusing at different energies, helping to identify the most efficient fusion reactions and conditions.
33. Why is helium-3 fusion considered advantageous despite its rarity on Earth?
Helium-3 fusion (with deuterium) is considered advantageous because it produces no neutrons, resulting in less radioactive waste and activation of reactor materials. It also allows for more direct conversion of fusion energy to electricity. However, helium-3 is extremely rare on Earth, making its use challenging. Some propose mining it from the Moon, where it's more abundant due to solar wind deposition.
34. How does the concept of nuclear isomers relate to energy release in nuclear reactions?
Nuclear isomers are excited states of atomic nuclei that have relatively long half-lives. In some cases, the energy difference between an isomer and its ground state can be released through a process called isomeric transition. This concept is relevant to both fission and fusion, as it represents another way nuclei can store and release energy, potentially affecting reaction products and energy output.
35. What is the role of plasma confinement in fusion reactions, and why isn't it a concern in fission?
Plasma confinement is crucial in fusion reactions because the fuel must be kept at extremely high temperatures and densities for sufficient time to allow fusion to occur. This is typically achieved using magnetic fields (in tokamaks) or inertial methods (in laser fusion). Fission doesn't require plasma confinement because it occurs with solid fuel at much lower temperatures, where traditional containment methods suffice.
36. Why is the triple-alpha process important in stellar nucleosynthesis, and how does it differ from typical fusion reactions?
The triple-alpha process is crucial in stellar nucleosynthesis as it bridges the gap between helium and carbon, allowing for the production of heavier elements. It differs from typical fusion reactions because it involves the simultaneous collision of three helium nuclei, which is much less probable than two-body collisions. This process only occurs at the high temperatures and densities found in the cores of aging stars.
37. How does the concept of nuclear deformation affect fission reactions?
Nuclear deformation refers to the departure of a nucleus from a spherical shape. In fission reactions, the degree of deformation affects the likelihood and products of fission. Highly deformed nuclei are more prone to fission. Understanding nuclear deformation helps predict fission fragment distributions and the energy released in fission reactions, which is crucial for reactor design and nuclear waste management.
38. What is the significance of the "valley of stability" in nuclear physics, and how does it relate to both fission and fusion?
The "valley of stability" is the region on the chart of nuclides where stable isotopes exist. It's shaped by the interplay between the strong nuclear force and electrostatic repulsion. In fission, products tend to move towards this valley through radioactive decay. In fusion, reactions generally move up the valley, creating more stable, heavier nuclei. Understanding this concept helps predict the behavior and products of nuclear reactions.
39. How does the presence of neutron poisons affect fission reactions, and is there an analogous concept in fusion?
Neutron poisons are elements with high neutron absorption cross-sections that can hinder fission chain reactions by absorbing neutrons without fissioning. They play a crucial role in reactor control and safety. In fusion, there's no direct analogue, but impurities in the plasma can cause energy loss through radiation, reducing fusion efficiency. Managing plasma purity is crucial for sustaining fusion reactions.
40. Why is the concept of breeding tritium important for future fusion reactors?
Breeding tritium is crucial for future fusion reactors because tritium, a key fuel component in D-T fusion, is radioactive with a short half-life and isn't naturally abundant. The concept involves using the neutrons produced in fusion reactions to create tritium from lithium in the reactor blanket. This process is essential for making fusion reactors self-sufficient in fuel production, addressing the limited availability of tritium.
41. How does the nuclear shell model explain "magic numbers" in nuclear stability, and how does this affect fission and fusion reactions?
The nuclear shell model, analogous to electron shells in atoms, explains the existence of "magic numbers" (2, 8, 20, 28, 50, 82, 126) of protons or neutrons that correspond to closed shells and increased nuclear stability. In fission, nuclei with magic numbers of protons or neutrons are more likely to form as fission products. In fusion, reaching these magic numbers can result in more stable products, affecting reaction rates and energy release.
42. What is the role of beta decay in the products of both fission and fusion reactions?
Beta decay plays a significant role in both fission and fusion products. In fission, many products are neutron-rich and undergo beta-minus decay to reach stability. In certain fusion reactions, such as proton-proton chain in stars, beta-plus decay (positron emission) occurs as part of the process. Understanding beta decay is crucial for predicting the behavior and half-lives of reaction products in both processes.
43. How does the concept of nuclear fission isomers relate to energy release in fission reactions?
Nuclear fission isomers are metastable states of certain heavy nuclei that can undergo fission. They represent an excited state of the nucleus that has a relatively long half-life before fissioning. This concept is important in understanding delayed neutron emission in fission reactions, which is crucial for reactor control. Fission isomers can affect the timing and energy release in fission processes.
44. Why is inertial confinement fusion challenging, and how does it differ from magnetic confinement fusion?
Inertial confinement fusion (ICF) is challenging because it requires precisely timed, extremely powerful laser pulses to compress and heat the fuel to fusion conditions in nanoseconds. It differs from magnetic confinement fusion (MCF) in that ICF relies on the inertia of the imploding fuel to provide confinement, while MCF uses magnetic fields to confine the plasma for longer periods. ICF aims for higher densities but shorter confinement times compared to MCF.