Light Energy - Definition, Uses, FAQs
We know that light energy is one of the forces that sustain life on earth since it is needed in natural processes such as photosynthesis and also used in technology such as solar energy. Whether it’s the rays of the sun that sustain ecosystems or advanced technologies such as lasers and fibres, light energy is an integral part of life today. As seen in the example, nature has been able to take something so simple and light in order to create something so powerful such as innovation, or rather clean energy.
JEE Main/NEET 2027: Physics Important Formulas for Class 10
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs

What is Light?
Light is nothing but electromagnetic radiation in which only the visible range of wavelength of electromagnetic radiation is visible to the human being. The instrument to measure the intensity of visible light is the photometer. Light is mentioned before as an electromagnetic wave which contains electric waves and magnetic waves which are perpendicular to each other, and the particle called photons which is a minute particle. Hence the speed of light is given by the equation as
Where c is the speed of light,
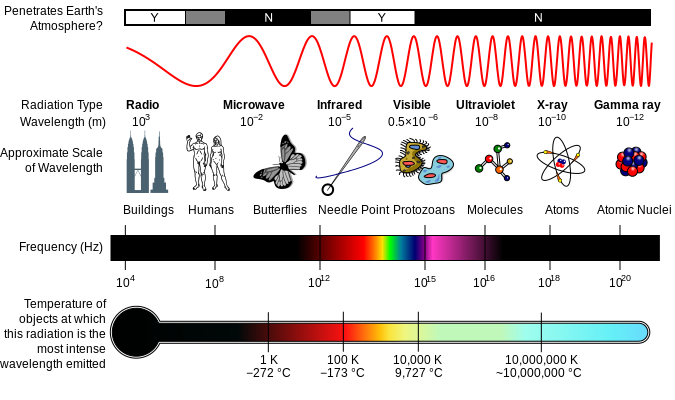
Radio waves
Microwaves
Infrared
Visible light
Ultraviolet waves
X-ray
Gamma waves
So the visible light is in the range of 10-6m. Light or visible light waves consist of photons that have no mass, the smallest quantity that can be transported. The light travels at a speed of 3×108m/s. The light from the sun’s surface takes 8 minutes and 17 seconds to reach the earth's surface. The interesting fact about light is that we human beings can’t travel at the speed of light, because only no mass particles like photons can travel at light speed. We can’t accelerate any object to travel at light speed, because it takes infinite energy to accelerate the objects. No matter is required to carry a light hence it can travel in space where no air is present. Unlike sound energy, it can travel only through objects like solids, liquids. So only sound can’t be heard in space.
Also, read
- NCERT Solutions for All Subjects
- NCERT Notes For All Subjects
- NCERT Exemplar Solutions for All Subjects
Types of Light Energy
There are three types of light energy:
1. Visible light.
2. Infrared light.
3. X-ray and UV light.
- Visible light
- It is the part of the electromagnetic spectrum that is visible to the human eye.
- Isaac Newton demonstrated through his prism experiment that white light can be split into 7 colours and form the basics of the colour system which exists today.
- It has frequencies of about
- The most important characteristic of the visible spectrum is "colour". It is both the inherent property of visible light and an important function of the human eye.
- Infrared light
- William Herschel discovered infrared light.
- When a warmer temperature is measured beyond the end of visible red light, infrared radiation is detected.
- It is the part of the electromagnetic spectrum that ranges from 800 nm to 1 mm and has a frequency of 300 GHz to 400 THz.
- Infrared radiation is emitted or absorbed by the molecules when they change their rotational and vibrational movements. The black body absorbs maximum infrared radiation.
- It is also responsible for detecting heat and is used in thermal imaging instruments.
- X-ray and UV light
- X-rays and UV light are the higher energy parts of the electromagnetic spectrum
- The wavelength of
- The frequency of
- Wilhelm Roentgen discovered the X-rays.
- X-rays are mainly used for medical and defence purposes.
What is VIBGYOR?
In the visible range of light waves of
Below is the wavelength of white light.
Violet 400 nm to 440 nm
Indigo 440 nm to 460 nm
Blue 460 nm to 500 nm
Green 500 nm to 570 nm
Yellow 570 nm to 590 nm
Orange 590 nm to 620 nm
Red 620 nm to 720 nm
The source of light in our day-to-day life is sunlight. The words related to light are photons stream, packets of energy, and flash. The antonyms for light are dark, glare, dull, and cloudy.
Uses of light
photosynthesis-food production by plants by using light
vitamin D - which is the essential vitamin for human being growth and skin complexion
vision – without light, no one can see the world.
Temperature- light is a form of energy. Sunlight, which is the source of light, maintains the world temperature by which proper photosynthesis happens, and weather and climatic conditions are maintained.
Sterilising agent- Ultraviolet rays kill microbes and keep things clean and sterile.
Solar energy- Light which is a form of solar energy is renewable energy. The electricity is produced from the solar panels which are inclined with an angle in sunlight by which PV cells are used to produce direct current.
Refraction – sunglasses, telescope, lens.
By using light we can also study the properties of matter. The matter is composed of atoms which are the smallest particles. Atoms have protons, electrons, and neutrons. Hence when the light intersects in the matter it gets altered because of the properties of matter. By studying the altered light we can learn about the material properties.
What is Light energy?
Light energy is a form of kinetic energy which can be transmitted without mass, as it is composed of minute particles called photons that have no mass. Sunlight emits sun rays which have electromagnetic rays in which only the visible range of wavelength of light is visible to the human eye. Now we can know how objects are getting to our eyes during the sunshine. We can see any object because light from the sun falls on the object, and it moves in space and gets reflected in our eyes. When the light reaches our eyes, the signals will be sent to the brain. The brain will derive the information from the signals that detect the object's size, shape, and presence. This signal transmission will happen in seconds.
Commercial uses of conversion of light energy
The conversion of light energy to chemical energy called photosynthesis is used by plants to prepare chlorophyll. When the photons from sunlight bombard an electron in the chloroplast, those electrons will get excited which has arisen in the energy levels. Chlorophyll absorbs only red light and green light from the white light and it reflects the green light to us. So only the colour of plants appears green to us.

Another commercial use of light energy is solar energy. Sunlight is the main source of power. Photovoltaic cells are used to convert sunlight to electricity. In simple words, solar energy uses the heat and light from the sun and converts that into direct current. When the sun's rays strike the panel energy from the sun is absorbed through the PV panels. This creates the flow of current in the panels which lights up the whole house. These solar panels when they are installed in each house can afford our own electricity demand. Unlike any energy like thermal energy, and nuclear energy this solar energy is free of pollution, maintenance-free, and renewable energy as it is available all over the year.

Frequently Asked Questions (FAQs)
The light travels at a speed of 3×108m/s. The light from the sun’s surface takes 8 minutes 17 seconds to reach the earth surface. As human beings, we can’t travel at the speed of light, because only no mass particles like photons can travel at light speed. We can’t accelerate any object to travel at light speed, because it takes infinite energy to accelerate the objects. No matter is required to carry a light hence it can travel in space where no air is present. Unlike sound energy, it can travel only through objects like solid, liquid. So only sound can’t be heard in space.
Light is a form of electromagnetic wave which contains electric waves and magnetic waves which are perpendicular to each other, and the particle called photons which is a minute particle.
Radio waves 103m
Microwaves 10-2m
Infrared 10-5m
Visible light 10-6m
Ultraviolet waves 10-8m
X-ray 10-10m
Gamma waves 10-12m
The visible range of light waves of 10-6m, the human eye can see the colours only in the 400nm to 700nm range. The colours are violet, indigo, blue, green, yellow, orange, red. The white light is the composition of seven different colours with a specific wavelength.
Below the wavelength of white light.
- Violet 400 nm to 440 nm
- Indigo 440 nm to 460 nm
- Blue 460 nm to 500 nm
- Green 500 nm to 570 nm
- Yellow 570 nm to 590 nm
- Orange 590 nm to 620 nm
- Red 620 nm to 720 nm
- Solar energy
- Photosynthesis
Also Read
02 Jul'25 06:05 PM
02 Jul'25 05:50 PM
02 Jul'25 05:31 PM
02 Jul'25 05:08 PM
02 Jul'25 05:02 PM
02 Jul'25 04:44 PM

