Electric Dipole - Definition, Formula, FAQs
Two charges, equal in magnitude, but opposite in sign separated by a small distance form a dipole. It is an elementary magnet whose positive end attracts negative ends while repelling positive ones; similarly, each side acts like a beginning and an end. Electric dipoles are essential for investigating how molecules interact since most of these compounds have such properties due to differences in their electron distribution between nuclei making them have a partial positive charge on one side while having partial negative charges on other angles of inclination towards this aspect instead if looked at it from different perspectives at the same time it makes it appear both as positive and negative but since they are near each other from the point at which we stand there is no way they could be seen. A torque is exerted on the dipole by the electric field; this torque tends to align the dipole with the field.
JEE Main/NEET 2027: Physics Important Formulas for Class 10
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Define Electric Dipole
- What is Electric Dipole Moment?
- Solved Examples Based on Electric Dipole
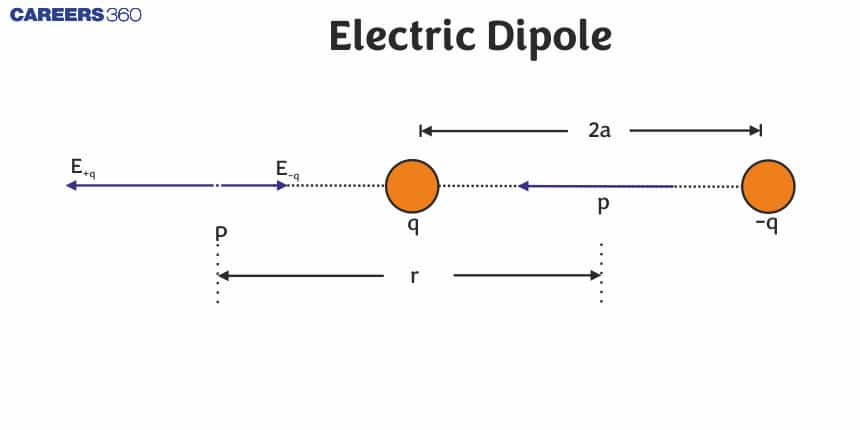
Define Electric Dipole
An electric dipole is a system of two equal and opposite point charges separated by a very small and finite distance.
Below is the figure showing an electric dipole consisting of two equal and opposite point charges

What is Electric Dipole Moment?
The strength of an electric dipole is measured by a vector quantity known as the electric dipole moment. Its magnitude is equal to the product of the magnitude of either charge and the distance between the two charges,
i.e. for the dipole, as shown in the above figure dipole moment is given as
And its direction is along the line from -q to +q.
The
Related Topics
Potential Due to an Electric Dipole
Consider an electric dipole, with charges
Case 1: When
Case 2: When
Also, read
Electric Field Due to an Electric Dipole
Coulomb's law and the superposition principle can be used to calculate the electric field of a pair of charges ( -q and q ) at any point in space. The results are particularly simple and clear for the following two scenarios.
The electric field is due to a dipole at any point (the line connecting the two charges).
Electric field due to dipole at equatorial point (a plane perpendicular to the dipole axis through its center).
Direction of Electric Dipole Moment
The electric dipole moment is a vector quantity. It has a defined direction which is from the negative charge to the positive charge. However, it is important to remember that this convention of direction is only followed in Physics.
Physical Significance of Dipole
A dipole has physical significance in the unique electric field generation, interaction with external fields, and contribution to polarization in materials. Chemically, it is behind molecular interactions; in physics, it describes dielectric properties; and is the basis for antenna technology emitting electromagnetic waves.
Solved Examples Based on Electric Dipole
Example 1: An electric dipole is placed in an electric field generated by a point charge
1) The net electric force on the dipole must be zero
2) The net electric force on the dipole may be zero
3) The torque on the dipole due to the field must be zero
4) The torque on the dipole due to the field may be zero
Solution:
Electric dipole
Two equal and opposite charges are separated by a small distance.
wherein

Point charge produces a non-uniform electric field
Example 2: A point dipole
1)
2)
3)
4)
Solution:

Example 3: Shown in the figure is a shell made of a conductor. It has an inner radius a and outer radius

1) surface charge density on the outer surface depends on
2) The electric field outside the shell is the same as that of a point charge at the centre of the shell
3) surface charge density on the inner surface is uniform and equal to
4) surface charge density on the inner surface of the shell is zero everywhere
Solution:
The charge distribution at equilibrium on the conductor will be like :

The net charge on the outer surface
The total charge on the inner surface
If we take a Gaussian surface outside the shell.
So net charge inside the Gaussian surface will be Q .
So far any observed outside the shell, the resultant electric field is due to
So electric field outside the shell is the same as that of a point charge at the centre of the shell.
Example 4: Two charges
1)
2)
3)
4)
Solution:
Dipole moment
Its S.I unit is C-m
and its CGS unit is Debye (
Dipole moment
Hence, the answer is the option (3).
Example 5: Two opposite and equal charges
1)
2)
3)
4)
Solution:
Given Data:
- Charge,
- Separation between charges,
- Electric field,
Step 1: Calculate the Dipole Moment (
The dipole moment
Substitute the values:
Step 2: Calculate the Maximum Torque (
The torque experienced by a dipole in a uniform electric field is given by:
For maximum torque,
Substitute the values:
To match the options, convert this to scientific notation:
Step 3: Calculate the Work Done in Rotating the Dipole through
The work done to rotate a dipole through an angle
For
Substitute the values:
To match the options, convert this to scientific notation:
Hence, the answer is the option (4).
Frequently Asked Questions (FAQs)
The forces acting on the dipole's two charges are equal and opposite. As a result, the net force is nil.
Coulomb.meter is the SI unit for dipole moment
The torque is greatest when the dipole is held perpendicular to the field.
The torque on a dipole is lowest when it is parallel to the field.
Also Read
02 Jul'25 05:01 PM
02 Jul'25 04:59 PM
02 Jul'25 04:58 PM
02 Jul'25 04:49 PM
02 Jul'25 04:46 PM
02 Jul'25 04:42 PM
02 Jul'25 04:40 PM
02 Jul'25 04:29 PM
