Access premium articles, webinars, resources to make the best decisions for career, course, exams, scholarships, study abroad and much more with
Plan, Prepare & Make the Best Career Choices
NCERT Solutions for Class 11 Physics Chapter 11 Thermal Properties of Matter
NCERT Solutions Class 11 Physics Chapter 11 – Access and Download For Free
NCERT Solutions for Class 11 Physics Chapter 11 Thermal Properties of Matter: If you're a Class 11 student seeking thermal properties of matter exercise solutions, you're in the right place for NCERT solutions. On this Careers360 page, you can access complete exercise solutions, covering questions from 11.1 to 11.20 in the main exercise, and questions 11.21 to 11.22 in the additional exercise. These accurate and precise class 11 physics chapter 11 exercise solutions are available for download in PDF format, providing convenient access for students. The answers have been crafted by the best subject matter experts, ensuring their reliability.
JEE Main Scholarship Test Kit (Class 11): Narayana | Physics Wallah | Aakash | Unacademy
NEET Scholarship Test Kit (Class 11): Narayana | Physics Wallah | Aakash | ALLEN
Suggested: JEE Main: high scoring chapters | Past 10 year's papers
New: Aakash iACST Scholarship Test. Up to 90% Scholarship. Register Now
- NCERT Solutions Class 11 Physics Chapter 11 – Access and Download For Free
- CERT Solutions for Class 11 Physics Chapter 11 Thermal Properties of Matter
- NCERT thermal properties of matter class 11 solutions - Additional Exercise Solution
- NCERT solutions for class 11 physics chapter wise
- Highlight of NCERT Solutions for Class 11 Physics Chapter 11 Thermal Properties of Matter
- More About Thermal Properties Of Matter Class 11 Physics Chapter 11
- NCERT solutions for class 11 Subject wise
Temperature is a relative measure of indication of coldness (or hotness) while heat is the form of energy transferred between two or more systems or a system and its surroundings due to the temperature difference. Thermal Properties of Matter Class 11 solutions of NCERT Physics Chapter start with the questions related to temperature conversions from one scale to another. All the questions discussed in the CBSE NCERT solutions for Class 11 Physics chapter 11 Thermal Properties of Matter are important to understand the topics covered in the chapter. ch 11 class 11 physics ncert solutions are an important tool to perform well in exams.
** This chapter has been renumbered as Chapter 10 in accordance with the CBSE Syllabus 2023–24.
Free download thermal properties of matter class 11 exercise solution PDF for CBSE exam.
CERT Solutions for Class 11 Physics Chapter 11 Thermal Properties of Matter
Access NCERT Solutions for Class 11 Physics Chapter 11 Thermal Properties of Matter - Exercise Solution
Answer:
The relation between Kelvin and Celcius scale is T K = T C + 273.15
Triple Point of Neon in Kelvin T K = 24.57 K
Triple Point of Neon in Celcius T C = T K -273.15 = 24.57 -273.15 =-248.58 o C
Triple Point of carbon dioxide in Kelvin T K = 216.55 K
Triple Point of carbon dioxide in Celcius T C = T K -273.15 = 216.55 - 273.15 = -56.60 o C
The relation between Celcius and Fahrenheit scale is
Triple Point of Neon in Fahrenheit is
T F = -415.44 o C
Triple Point of carbon dioxide in Fahrenheit is
T F = -69.88 o C
Answer:
200 A = 273 K
350 B = 273 K
Equating T K From the above two equations we have
The resistance is at the triple-point of water
and
at the normal melting point of lead
What is the temperature when the resistance is
?
Answer:
R 0 = T 0 =
R = R =
Putting the above values in the given equation we have
For R =
Answer:
Unlike the melting point of ice and boiling point of water, the triple point of water has a fixed value of 273.16 K. The melting point of ice and boiling point of water vary with pressure.
Q. 11.4 (b) Answer the following :
Answer:
The other fixed point on the Kelvin scale is 0 K. 0K is the absolute zero
Q. 11.4 (c) Answer the following :
(c) The absolute temperature (Kelvin scale) is related to the temperature
on the Celsius scale by
Why do we have in this relation, and not
Answer:
This is because 0 o C on the Celcius scale corresponding to the melting point at standard pressure is equal to 273.15 K whereas 273.16 K is the triple point of water. The triple point of water is 0.01 0 C , not 0 0 C
Q. 11.4 (d) Answer the following :
Answer:
Let at a certain temperature the reading on Fahrenheit and Kelvin Scale be T F and T K respectively
Let at another temperature the reading on Fahrenheit and Kelvin Scale be T' F and T' K respectively
Subtracting equation (ii) from (i)
For T K - T' K = 1 K, T F - T' F = 9/5
Therefore corresponding to 273.16 K the absolute scale whose unit interval size is equal to that of the Fahrenheit scale
Temperature | Pressure Thermometer A | Pressure Thermometer B |
Triple-point of water | ||
Normal melting point of sulphur |
(a) What is the absolute temperature of normal melting point of sulphur as read by thermometers A and B?
Answer:
As the moles of oxygen and hydrogen inside the thermometers and the volume occupied by the gases remain constant P/T would remain constant.
The triple point of water(T 1 ) = 273.16 K
Pressure in thermometer A at a temperature equal to the triple point of water (P 1 ) =
Pressure in thermometer A at a temperature equal to Normal melting point of sulphur (P 2 ) =
The normal melting point of sulphur as read by thermometer A T 2 would be given as
Pressure in thermometer B at a temperature equal to the triple point of water (P' 1 ) =
Pressure in thermometer B at a temperature equal to Normal melting point of sulphur (P' 2 ) =
The normal melting point of sulphur as read by thermometer B T' 2 would be given as
Temperature | Pressure Thermometer A | Pressure Thermometer B |
Triple-point of water | ||
Normal melting point of sulphur |
(b) What do you think is the reason behind the slight difference in answers of thermometers A and B? (The thermometers are not faulty). What further procedure is needed in the experiment to reduce the discrepancy between the two readings?
Answer:
The slight difference in answers of thermometers A and B occur because the gases used in the thermometers are not ideal gases. To reduce this discrepancy the experiments should be carried out at low pressures where the behaviour of real gases tend close to that of ideal gases.
Answer:
At 27 o C the 63 cm (l 1 ) mark on the steel tape would be measuring exactly 63 cm as the tape is calibrated at 27 o C
Coefficient of linear expansion of steel
Actual length when the scale is giving a reading of 63 cm on at 45 o C is l 2
The actual length of the steel rod on a day when the temperature is 45 o C is 63.013608 cm.
Length of the same steel rod on a day when the temperature is 63 cm.
Answer:
Diameter of the steel shaft at 27 o C (T 1 ) d 1 = 8.70 cm
The diameter of the central hole in the wheel d 2 = 8.69 cm
Coefficient of linear expansion of the steel .
The wheel will slip on the shaft when the diameter of the steel shaft becomes equal to the diameter of the central hole in the wheel.
Let this happen at temperature T
Answer:
Coefficient of linear expansion of copper
Coefficient of superficial expansion of copper is
Diameter of the hole at 27 o C (d 1 ) = 4.24 cm
Area of the hole at 227 o C is
Let the diameter at 227 o C be d 2
Change in diameter is d 2 -d 1 = 4.24 -4.254 = 0.014 cm.
Answer:
Youngs Modulus of Brass,
Co-efficient of linear expansion of Brass,
The diameter of the given brass wire, d = 2.0 mm
Length of the given brass wire, l = 1.8 m
Initial Temperature T 1 = 27 o C
Final Temperature T 2 = -39 o C
The tension developed in the wire is 378 N. The negative sign signifies this tension is inward.
Answer:
Length of the rods l = 50 cm
Co-efficient of linear expansion of brass,
Co-efficient of linear expansion of steel,
Initial Temperature T 1 = 40.0 o C
Final Temperature T 2 = 250 o C
Change in length of brass rod is
Change in length of the steel rod is
Change in length of the combined rod is
Answer:
Coefficient of volume expansion of glycerine is
Let initial volume and mass of a certain amount of glycerine be V and m respectively.
Initial density is
Change in volume for a 30 o C rise in temperature will be
Final Density is
Fractional Change in density is
The negative sign signifies with an increase in temperature density will decrease.
Answer:
Power of the drilling machine, P = 10 kW
Time. t = 2.5 min
Total energy dissipated E is
Thermal energy absorbed by aluminium block is
Mass of the aluminium block, m = 8.0 kg
Specific heat of aluminium, c = 0.91 J g -1 K -1
Let rise in temperature be
Answer:
Mass of copper block m = 2.5 kg
Initial Temperature of the copper block, T 1 = 500 o C
Final Temperature of Copper block, T 2 = 0 o C
Specific heat of copper, c = 0.39 J g -1 K -1
Thermal Energy released by the copper block is
Latent heat of fusion of water, L = 335 j g -1
Amount of ice that can melt is
1.455 kg of ice can melt using the heat released by the copper block.
Answer:
Let the specific heat of the metal be C.
Mass of metal block m = 200 g
Initial Temperature of metal block = 150 o C
Final Temperature of metal block = 40 o C
The heat released by the block is
Initial Temperature of the calorimeter and water = 27 o C
Final Temperature of the calorimeter and water = 40 o C
Amount of water = 150 cm
Mass of water = 150 g
Water equivalent of calorimeter = 25 g
Specific heat of water = 4.186 J g -1 K -1
Heat absorbed by the Calorimeter and water is
The heat absorbed by the Calorimeter and water is equal to the heat released by the block
The above value would be lesser than the actual value since some heat must have been lost to the surroundings as well which we haven't accounted for.
Q. 11.15 Given below are observations on molar specific heats at room temperature of some common gases.
Gas Molar specific heat (Cv )
Hydrogen 4.87
Nitrogen 4.97
Oxygen 5.02
Nitric oxide 4.99
Carbon monoxide 5.01
Chlorine 6.17
The measured molar specific heats of these gases are markedly different from those for monatomic gases. Typically, molar specific heat of a monatomic gas is Explain this difference. What can you infer from the somewhat larger (than the rest) value for chlorine?
Answer:
Monoatomic gases have only translational degree of freedom but diatomic gases have rotational degrees of freedom as well. The temperature increases with increase in the spontaneity of motion in all degrees. Therefore to increase the temperature of diatomic gases more energy is required than that required to increase the temperature of monoatomic gases by the same value owing to higher degrees of freedom in diatomic gases.
If we only consider rotational modes of freedom the molar specific heat of the diatomic gases would be given as
The number of degrees of freedom = 5 (3 translational and 2 rotational)
The values given in the table are more or less in accordance with the above calculated one. The larger deviation from the calculated value in the case of chlorine is because of the presence of vibrational motion as well.
Answer:
Initial Temperature of the boy = 101 o F
Final Temperature of the boy = 98 o F
Change in Temperature is
Mass of the child is m = 30 kg
Specific heat of human body = 1000 cal kg -1 o C -1
Heat released is
Latent heat of evaporation of water = 580 cal g -1
The amount of heat lost by the body of the boy has been absorbed by water.
Let the mass of water which has evaporated be m'
Time in which the water has evaporated, t = 20 min.
Rate of evaporation is m'/t
Answer:
Side of the box s = 30 cm
Area available for conduction A
A = 6s 2
A=6(30) 2
A=5400 cm 2 = 0.54 m 2
Temperature difference = 45 o C
Co-efficient of thermal conductivity of thermacole is k = 0.01 J s -1 m -1 K -1
Width of the box is d = 5 cm
Heat absorbed by the box in 6 hours is
The heat of fusion of water is
Amount of ice which has melted is m'
Amount of ice left after 6 hours = 4 - 0.313 = 3.687 kg
Answer:
The rate at which water boils, R = 6.0 kg min -1
The heat of vaporisation of water,
The rate at which heat enters the boiler
The base area of the boiler, A = 0.15 m 2
Thickness, l = 1.0 cm
Thermal conductivity of brass
The temperature inside the boiler = Boiling point of water = 100 o C
Let the temperature of the flame in contact with the boiler be T
Amount of heat flowing into the boiler is
The temperature of the flame in contact with the boiler is 237.98 o C
Q. 11.19 (a) Explain why :
(a) a body with large reflectivity is a poor emitter
Answer:
A body with a large reflectivity is a poor absorber. As we know a body which is a poor absorber will as well be a poor emitter. Therefore a body with large reflectivity is a poor emitter.
Q .11.19 (b) Explain why :
(b) a brass tumbler feels much colder than a wooden tray on a chilly day
Answer:
Brass is a good conductor of heat. Therefore once someone touches brass heat from their body flows into it and it feels cold, in case of a wooden tray, no such conduction of heat from the body takes place as wood is a very poor conductor of heat.
Q. 11.19 (c) Explain why :
Answer:
An optical pyrometer relates the brightness of a glowing body with its temperature. In the open because of other sources of light the sensor in the optical pyrometer does not detect the true brightness of a red hot piece of iron and thus does not predict its temperature correctly whereas in the furnace the piece of iron is the only source of light and the sensor detects its brightness correctly thus giving the correct value of the temperature.
Q. 11.19 (d) Explain why :
(d) the earth without its atmosphere would be inhospitably cold
Answer:
The sun rays contain infrared radiations. These are reflected back by the lower part of the atmosphere after being reflected by the surface of the earth and are trapped inside the atmosphere thus maintaining the Earth's temperature at a hospitable level. Without these rays being trapped the temperature of the earth will go down severely and thus the Earth without its atmosphere would be inhospitably cold.
Q. 11.19 (e) Explain why :
Answer:
Heating systems based on the circulation of steam are more efficient in warming a building than those based on the circulation of hot water because the same amount of steam at 100 o C contains more energy available for heat dissipation than the same amount of water at 100 o C in the form of latent heat of vaporization.
Answer:
Let a body initially be at temperature T 1
Let its final Temperature be T 2
Let the surrounding temperature be T 0
Let the temperature change in time t.
According to Newton's Law of cooling
where K is a constant.
We have been given that the body cools from 80 o C to 50 o C in 5 minutes when the surrounding temperature is 20 o C.
T 2 = 50 o C
T 1 = 80 o C
T 0 = 20 o C
t = 5 min = 300 s.
For T 1 = 60 o C and T 2 = 30 o C we have
The body will take 10 minutes to cool from 60 o C to 30 o C at the surrounding temperature of 20 o C.
NCERT thermal properties of matter class 11 solutions - Additional Exercise Solution
Q. 11.21(a) Answer the following questions based on the P-T phase diagram of carbon dioxide:
(a) At what temperature and pressure can the solid, liquid and vapour phases of co-exist in equilibrium?
Answer:
At the triple point temperature of -56.6 o C and pressure 5.11 atm the solid, liquid and vapour phases of co-exist in equilibrium.
Q. 11.21(b) Answer the following questions based on the P-T phase diagram of carbon dioxide:
(b) What is the effect of decrease of pressure on the fusion and boiling point of ?
Answer:
Both fusion and boiling point of CO 2 decrease with decrease in pressure. This we can see from the solid lines in the P-T phase diagram of CO 2 .
Q. 11.21 (c) Answer the following questions based on the P-T phase diagram of carbon dioxide:
(c) What are the critical temperature and pressure for ? What is their significance?
Answer:
The critical temperature and pressure for CO 2 are 31.1 o C and 73.0 atm respectively. If the temperature exceeds this critical value of temperature CO 2 would not liquefy no matter how high the pressure is.
Q .11.21 d(a) Answer the following questions based on the P-T phase diagram of carbon dioxide:
(d) Is solid, liquid or gas at
(a) under
Answer:
CO 2 is vapour at -70 o C under 1 atm pressure as the point corresponding to this condition lies in vapour region in the given P-T phase diagram of carbon dioxide.
Q .11.21 d (b) Answer the following questions based on the P-T phase diagram of carbon dioxide:
(d) Is solid, liquid or gas at
(b) under
Answer:
CO 2 is solid at -60 o C under 10 atm pressure as the point corresponding to this condition lies in the solid region in the given P-T phase diagram of carbon dioxide.
Q. 11.21 d(c) Answer the following questions based on the P-T phase diagram of carbon dioxide:
(d) Is solid, liquid or gas at
(c) under
Answer:
CO 2 is liquid at 15 o C under 56 atm pressure as the point corresponding to this condition lies in the liquid region in the given P-T phase diagram of carbon dioxide.
Q. 11.22 (a) Answer the following questions based on the P – T phase diagram of :
(a) at 1 atm pressure and temperature
is compressed isothermally. Does it go through a liquid phase?
Answer:
The temperature -60 o C lies to the left of the triple point of water i.e. in the region of solid and vapour phases. Once we start compressing CO 2 at this temperature starting from 1 atm pressure it will directly convert into solid without going through the liquid phase.
Q. 11.22 (b) Answer the following questions based on the P – T phase diagram of :
(b) What happens when at
atm pressure is cooled from room temperature at constant pressure?

Answer:
At room temperature (27 o C) and 4 atm pressure CO 2 exits in the vapour phase. The pressure 4 atm is less than the pressure at the triple point and therefore points corresponding to all temperatures and this pressure lie in the solid and vapour region. Once we start compressing CO 2 from room temperature at this constant pressure CO 2 turns from vapour to solid directly without going through the liquid phase.
Q. 11.22 (c) Answer the following questions based on the P – T phase diagram of :
(c) Describe qualitatively the changes in a given mass of solid at
pressure and temperature
as it is heated up to room temperature at constant pressure.
Answer:
At -65 o C under 10 atm pressure CO 2 is in the solid phase. At room temperature (27 o C) under 10 atm pressure CO 2 is in the vapour phase. At 10 atm pressure, CO 2 can exist in all three phases depending upon the temperature. Therefore as CO 2 is heated from -65 o C to room temperature at a constant pressure of 10 atm it goes from the solid phase to liquid phase and then ultimately it goes into the vapour phase.
Q. 11.22 (d) Answer the following questions based on the P – T phase diagram of :
(d) is heated to a temperature
and compressed isothermally. What changes in its properties do you expect to observe?
Answer:
70 o C is above the critical temperature of CO 2 . Once CO 2 is isothermally compressed at this temperature it would not liquefy irrespective of how high the pressure is but at very high pressures CO 2 will not behave as an ideal gas.
The thermal properties of matter class 11 exercise solution are essential for board exams because questions from this chapter often appear. These solutions are also crucial for competitive exams like JEE and NEET, where a good understanding of thermal properties is frequently tested, impacting students' overall performance and future career opportunities. Also, mastering this chapter is important because it forms the basis for more advanced physics topics and is closely connected to other chapters, particularly those related to thermodynamics.
NCERT solutions for class 11 physics chapter wise
Chapter 1 | |
Chapter 2 | |
Chapter 3 | |
Chapter 4 | |
Chapter 5 | |
Chapter 6 | |
Chapter 7 | |
Chapter 8 | |
Chapter 9 | |
Chapter 10 | |
Chapter 11 | Thermal Properties of Matter |
Chapter 12 | |
Chapter 13 | |
Chapter 14 | |
Chapter 15 |
Thermal properties of matter class 11 solutions: Important Formulas and Diagrams
Modulus of rigidity
![]()
Poisson’s ratio
![]()
Elastic energy
U = 1/2*strain*stress*volume
Heat Capacity
![]()
Where:
ΔQ is the amount of heat supplied to the substance and ΔT change in its temperature
Specific heat capacity
![]()
These formulas and diagrams are valuable tools for students as they help in solving complex problems related to thermal properties of matter, aiding in a deeper comprehension of the subject and enhancing problem-solving skills
Highlight of NCERT Solutions for Class 11 Physics Chapter 11 Thermal Properties of Matter
Highlights of thermal properties of matter class 11 ncert solutions are given below:
In ch 11 class 11 physics ncert solutions pdf, points are used to frame answers to help understand quickly.
Solution for thermal properties of matter class 11 are derived from the textbook by a highly qualified subject matter expert.
Questions and answers for ch 11 physics class 11 are given as per the latest CBSE Syllabus and guidelines.
thermal properties of matter class 11 ncert pdf links are readily available and easily accessible for free.
class 11 physics chapter 11 exercise solutions also boosts your knowledge and interest in physics. NCERT is the base of your learning and here it's easy to access.
More About Thermal Properties Of Matter Class 11 Physics Chapter 11
Thermal Properties of Matter Class 11 NCERT Topics
11.2 Temperature and heat
11.3 Measurement of temperature
11.4 Ideal-gas equation and absolute temperature
11.5 Thermal expansion
11.6 Specific heat capacity
11.7 Calorimetry
11.8 Change of state
11.9 Heat transfer
11.10 Newton’s law of cooling
Temperature And Heat
The difference between temperature and heat can be well understood from the following graph given in NCERT.
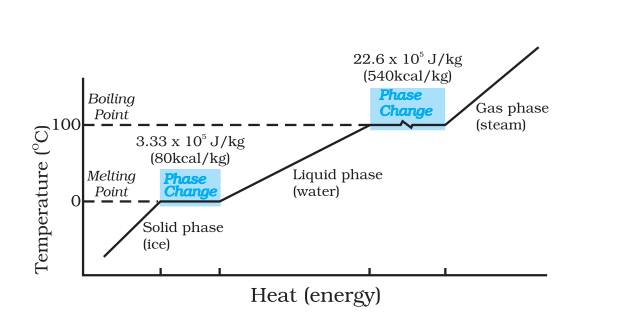
Even though heat is increased during the phase change, the temperature remains constant until the phase change has occurred. That is increasing heat always does not imply that there is an increase in temperature.
Dropped Topics –
The topics "Greenhouse Effect" and exercises 11.21 to 11.22 have been excluded from the ch 11 class 11 physics ncert solutions, "Thermal Properties of Matter," implying that they may not be part of the prescribed curriculum or are optional for students.
NCERT solutions for class 11 Subject wise
Also Check NCERT Books and NCERT Syllabus here
- NCERT Books Class 11 Physics
- NCERT Syllabus Class 11 Physics
- NCERT Books Class 11
- NCERT Syllabus Class 11
Subject wise NCERT Exemplar solutions
Frequently Asked Question (FAQs)
One question can be expected for NEET exam from the chapter Thermal Properties of Matter. Students can practice more questions from previous year NEET papers.
One question may be asked from Class 11 Physics chapter 11 Thermal Properties of Matter for JEE Main exam. This is one important chapter for KVPY and NSEP exms. Students can get more problems on the chapter from the NCERT Exemplar.
Topics covered in NCERT book Class 11 chapter Thermal Properties of Matter are-
- Temperature and heat
- Measurement of temperature
- Ideal-gas equation and absolute temperature
- Thermal expansion
- Specific heat capacity
- Calorimetry
- Change of state
- Heat transfer
- Newton’s law of cooling
One reliable online source to access thermal properties of matter ncert solutions is Careers360. These solutions have been created by subject experts to aid students in their exam preparation. The solutions provide clear and easy-to-understand explanations to help students gain a better conceptual understanding. The solutions are available in PDF format, making it convenient for students to access and save time while finding the correct answers.
There are a total of 10 topics in this chapter.
In the Class 11 Physics Chapter 11, "Thermal Properties of Matter," exercise, there are typically 22 questions are present, out of 22 questions from 1-20( exercise) and 21-22( additional exercise).
Also Read
Articles
Popular Questions
Colleges After 12th
Popular Course After 12th
Explore Career Options (By Industry)
Data Administrator
Database professionals use software to store and organise data such as financial information, and customer shipping records. Individuals who opt for a career as data administrators ensure that data is available for users and secured from unauthorised sales. DB administrators may work in various types of industries. It may involve computer systems design, service firms, insurance companies, banks and hospitals.
Bio Medical Engineer
The field of biomedical engineering opens up a universe of expert chances. An Individual in the biomedical engineering career path work in the field of engineering as well as medicine, in order to find out solutions to common problems of the two fields. The biomedical engineering job opportunities are to collaborate with doctors and researchers to develop medical systems, equipment, or devices that can solve clinical problems. Here we will be discussing jobs after biomedical engineering, how to get a job in biomedical engineering, biomedical engineering scope, and salary.
Ethical Hacker
A career as ethical hacker involves various challenges and provides lucrative opportunities in the digital era where every giant business and startup owns its cyberspace on the world wide web. Individuals in the ethical hacker career path try to find the vulnerabilities in the cyber system to get its authority. If he or she succeeds in it then he or she gets its illegal authority. Individuals in the ethical hacker career path then steal information or delete the file that could affect the business, functioning, or services of the organization.
GIS Expert
GIS officer work on various GIS software to conduct a study and gather spatial and non-spatial information. GIS experts update the GIS data and maintain it. The databases include aerial or satellite imagery, latitudinal and longitudinal coordinates, and manually digitized images of maps. In a career as GIS expert, one is responsible for creating online and mobile maps.
Data Analyst
The invention of the database has given fresh breath to the people involved in the data analytics career path. Analysis refers to splitting up a whole into its individual components for individual analysis. Data analysis is a method through which raw data are processed and transformed into information that would be beneficial for user strategic thinking.
Data are collected and examined to respond to questions, evaluate hypotheses or contradict theories. It is a tool for analyzing, transforming, modeling, and arranging data with useful knowledge, to assist in decision-making and methods, encompassing various strategies, and is used in different fields of business, research, and social science.
Geothermal Engineer
Individuals who opt for a career as geothermal engineers are the professionals involved in the processing of geothermal energy. The responsibilities of geothermal engineers may vary depending on the workplace location. Those who work in fields design facilities to process and distribute geothermal energy. They oversee the functioning of machinery used in the field.
Database Architect
If you are intrigued by the programming world and are interested in developing communications networks then a career as database architect may be a good option for you. Data architect roles and responsibilities include building design models for data communication networks. Wide Area Networks (WANs), local area networks (LANs), and intranets are included in the database networks. It is expected that database architects will have in-depth knowledge of a company's business to develop a network to fulfil the requirements of the organisation. Stay tuned as we look at the larger picture and give you more information on what is db architecture, why you should pursue database architecture, what to expect from such a degree and what your job opportunities will be after graduation. Here, we will be discussing how to become a data architect. Students can visit NIT Trichy, IIT Kharagpur, JMI New Delhi.
Remote Sensing Technician
Individuals who opt for a career as a remote sensing technician possess unique personalities. Remote sensing analysts seem to be rational human beings, they are strong, independent, persistent, sincere, realistic and resourceful. Some of them are analytical as well, which means they are intelligent, introspective and inquisitive.
Remote sensing scientists use remote sensing technology to support scientists in fields such as community planning, flight planning or the management of natural resources. Analysing data collected from aircraft, satellites or ground-based platforms using statistical analysis software, image analysis software or Geographic Information Systems (GIS) is a significant part of their work. Do you want to learn how to become remote sensing technician? There's no need to be concerned; we've devised a simple remote sensing technician career path for you. Scroll through the pages and read.
Budget Analyst
Budget analysis, in a nutshell, entails thoroughly analyzing the details of a financial budget. The budget analysis aims to better understand and manage revenue. Budget analysts assist in the achievement of financial targets, the preservation of profitability, and the pursuit of long-term growth for a business. Budget analysts generally have a bachelor's degree in accounting, finance, economics, or a closely related field. Knowledge of Financial Management is of prime importance in this career.
Data Analyst
The invention of the database has given fresh breath to the people involved in the data analytics career path. Analysis refers to splitting up a whole into its individual components for individual analysis. Data analysis is a method through which raw data are processed and transformed into information that would be beneficial for user strategic thinking.
Data are collected and examined to respond to questions, evaluate hypotheses or contradict theories. It is a tool for analyzing, transforming, modeling, and arranging data with useful knowledge, to assist in decision-making and methods, encompassing various strategies, and is used in different fields of business, research, and social science.
Underwriter
An underwriter is a person who assesses and evaluates the risk of insurance in his or her field like mortgage, loan, health policy, investment, and so on and so forth. The underwriter career path does involve risks as analysing the risks means finding out if there is a way for the insurance underwriter jobs to recover the money from its clients. If the risk turns out to be too much for the company then in the future it is an underwriter who will be held accountable for it. Therefore, one must carry out his or her job with a lot of attention and diligence.
Product Manager
A Product Manager is a professional responsible for product planning and marketing. He or she manages the product throughout the Product Life Cycle, gathering and prioritising the product. A product manager job description includes defining the product vision and working closely with team members of other departments to deliver winning products.
Operations Manager
Individuals in the operations manager jobs are responsible for ensuring the efficiency of each department to acquire its optimal goal. They plan the use of resources and distribution of materials. The operations manager's job description includes managing budgets, negotiating contracts, and performing administrative tasks.
Stock Analyst
Individuals who opt for a career as a stock analyst examine the company's investments makes decisions and keep track of financial securities. The nature of such investments will differ from one business to the next. Individuals in the stock analyst career use data mining to forecast a company's profits and revenues, advise clients on whether to buy or sell, participate in seminars, and discussing financial matters with executives and evaluate annual reports.
Researcher
A Researcher is a professional who is responsible for collecting data and information by reviewing the literature and conducting experiments and surveys. He or she uses various methodological processes to provide accurate data and information that is utilised by academicians and other industry professionals. Here, we will discuss what is a researcher, the researcher's salary, types of researchers.
Welding Engineer
Welding Engineer Job Description: A Welding Engineer work involves managing welding projects and supervising welding teams. He or she is responsible for reviewing welding procedures, processes and documentation. A career as Welding Engineer involves conducting failure analyses and causes on welding issues.
Transportation Planner
A career as Transportation Planner requires technical application of science and technology in engineering, particularly the concepts, equipment and technologies involved in the production of products and services. In fields like land use, infrastructure review, ecological standards and street design, he or she considers issues of health, environment and performance. A Transportation Planner assigns resources for implementing and designing programmes. He or she is responsible for assessing needs, preparing plans and forecasts and compliance with regulations.
Environmental Engineer
Individuals who opt for a career as an environmental engineer are construction professionals who utilise the skills and knowledge of biology, soil science, chemistry and the concept of engineering to design and develop projects that serve as solutions to various environmental problems.
Safety Manager
A Safety Manager is a professional responsible for employee’s safety at work. He or she plans, implements and oversees the company’s employee safety. A Safety Manager ensures compliance and adherence to Occupational Health and Safety (OHS) guidelines.
Conservation Architect
A Conservation Architect is a professional responsible for conserving and restoring buildings or monuments having a historic value. He or she applies techniques to document and stabilise the object’s state without any further damage. A Conservation Architect restores the monuments and heritage buildings to bring them back to their original state.
Structural Engineer
A Structural Engineer designs buildings, bridges, and other related structures. He or she analyzes the structures and makes sure the structures are strong enough to be used by the people. A career as a Structural Engineer requires working in the construction process. It comes under the civil engineering discipline. A Structure Engineer creates structural models with the help of computer-aided design software.
Highway Engineer
Highway Engineer Job Description: A Highway Engineer is a civil engineer who specialises in planning and building thousands of miles of roads that support connectivity and allow transportation across the country. He or she ensures that traffic management schemes are effectively planned concerning economic sustainability and successful implementation.
Field Surveyor
Are you searching for a Field Surveyor Job Description? A Field Surveyor is a professional responsible for conducting field surveys for various places or geographical conditions. He or she collects the required data and information as per the instructions given by senior officials.
Orthotist and Prosthetist
Orthotists and Prosthetists are professionals who provide aid to patients with disabilities. They fix them to artificial limbs (prosthetics) and help them to regain stability. There are times when people lose their limbs in an accident. In some other occasions, they are born without a limb or orthopaedic impairment. Orthotists and prosthetists play a crucial role in their lives with fixing them to assistive devices and provide mobility.
Pathologist
A career in pathology in India is filled with several responsibilities as it is a medical branch and affects human lives. The demand for pathologists has been increasing over the past few years as people are getting more aware of different diseases. Not only that, but an increase in population and lifestyle changes have also contributed to the increase in a pathologist’s demand. The pathology careers provide an extremely huge number of opportunities and if you want to be a part of the medical field you can consider being a pathologist. If you want to know more about a career in pathology in India then continue reading this article.
Gynaecologist
Gynaecology can be defined as the study of the female body. The job outlook for gynaecology is excellent since there is evergreen demand for one because of their responsibility of dealing with not only women’s health but also fertility and pregnancy issues. Although most women prefer to have a women obstetrician gynaecologist as their doctor, men also explore a career as a gynaecologist and there are ample amounts of male doctors in the field who are gynaecologists and aid women during delivery and childbirth.
Audiologist
The audiologist career involves audiology professionals who are responsible to treat hearing loss and proactively preventing the relevant damage. Individuals who opt for a career as an audiologist use various testing strategies with the aim to determine if someone has a normal sensitivity to sounds or not. After the identification of hearing loss, a hearing doctor is required to determine which sections of the hearing are affected, to what extent they are affected, and where the wound causing the hearing loss is found. As soon as the hearing loss is identified, the patients are provided with recommendations for interventions and rehabilitation such as hearing aids, cochlear implants, and appropriate medical referrals. While audiology is a branch of science that studies and researches hearing, balance, and related disorders.
Oncologist
An oncologist is a specialised doctor responsible for providing medical care to patients diagnosed with cancer. He or she uses several therapies to control the cancer and its effect on the human body such as chemotherapy, immunotherapy, radiation therapy and biopsy. An oncologist designs a treatment plan based on a pathology report after diagnosing the type of cancer and where it is spreading inside the body.
Anatomist
Are you searching for an ‘Anatomist job description’? An Anatomist is a research professional who applies the laws of biological science to determine the ability of bodies of various living organisms including animals and humans to regenerate the damaged or destroyed organs. If you want to know what does an anatomist do, then read the entire article, where we will answer all your questions.
Actor
For an individual who opts for a career as an actor, the primary responsibility is to completely speak to the character he or she is playing and to persuade the crowd that the character is genuine by connecting with them and bringing them into the story. This applies to significant roles and littler parts, as all roles join to make an effective creation. Here in this article, we will discuss how to become an actor in India, actor exams, actor salary in India, and actor jobs.
Acrobat
Individuals who opt for a career as acrobats create and direct original routines for themselves, in addition to developing interpretations of existing routines. The work of circus acrobats can be seen in a variety of performance settings, including circus, reality shows, sports events like the Olympics, movies and commercials. Individuals who opt for a career as acrobats must be prepared to face rejections and intermittent periods of work. The creativity of acrobats may extend to other aspects of the performance. For example, acrobats in the circus may work with gym trainers, celebrities or collaborate with other professionals to enhance such performance elements as costume and or maybe at the teaching end of the career.
Video Game Designer
Career as a video game designer is filled with excitement as well as responsibilities. A video game designer is someone who is involved in the process of creating a game from day one. He or she is responsible for fulfilling duties like designing the character of the game, the several levels involved, plot, art and similar other elements. Individuals who opt for a career as a video game designer may also write the codes for the game using different programming languages.
Depending on the video game designer job description and experience they may also have to lead a team and do the early testing of the game in order to suggest changes and find loopholes.
Radio Jockey
Radio Jockey is an exciting, promising career and a great challenge for music lovers. If you are really interested in a career as radio jockey, then it is very important for an RJ to have an automatic, fun, and friendly personality. If you want to get a job done in this field, a strong command of the language and a good voice are always good things. Apart from this, in order to be a good radio jockey, you will also listen to good radio jockeys so that you can understand their style and later make your own by practicing.
A career as radio jockey has a lot to offer to deserving candidates. If you want to know more about a career as radio jockey, and how to become a radio jockey then continue reading the article.
Choreographer
The word “choreography" actually comes from Greek words that mean “dance writing." Individuals who opt for a career as a choreographer create and direct original dances, in addition to developing interpretations of existing dances. A Choreographer dances and utilises his or her creativity in other aspects of dance performance. For example, he or she may work with the music director to select music or collaborate with other famous choreographers to enhance such performance elements as lighting, costume and set design.
Social Media Manager
A career as social media manager involves implementing the company’s or brand’s marketing plan across all social media channels. Social media managers help in building or improving a brand’s or a company’s website traffic, build brand awareness, create and implement marketing and brand strategy. Social media managers are key to important social communication as well.
Photographer
Photography is considered both a science and an art, an artistic means of expression in which the camera replaces the pen. In a career as a photographer, an individual is hired to capture the moments of public and private events, such as press conferences or weddings, or may also work inside a studio, where people go to get their picture clicked. Photography is divided into many streams each generating numerous career opportunities in photography. With the boom in advertising, media, and the fashion industry, photography has emerged as a lucrative and thrilling career option for many Indian youths.
Producer
An individual who is pursuing a career as a producer is responsible for managing the business aspects of production. They are involved in each aspect of production from its inception to deception. Famous movie producers review the script, recommend changes and visualise the story.
They are responsible for overseeing the finance involved in the project and distributing the film for broadcasting on various platforms. A career as a producer is quite fulfilling as well as exhaustive in terms of playing different roles in order for a production to be successful. Famous movie producers are responsible for hiring creative and technical personnel on contract basis.
Copy Writer
In a career as a copywriter, one has to consult with the client and understand the brief well. A career as a copywriter has a lot to offer to deserving candidates. Several new mediums of advertising are opening therefore making it a lucrative career choice. Students can pursue various copywriter courses such as Journalism, Advertising, Marketing Management. Here, we have discussed how to become a freelance copywriter, copywriter career path, how to become a copywriter in India, and copywriting career outlook.
Vlogger
In a career as a vlogger, one generally works for himself or herself. However, once an individual has gained viewership there are several brands and companies that approach them for paid collaboration. It is one of those fields where an individual can earn well while following his or her passion.
Ever since internet costs got reduced the viewership for these types of content has increased on a large scale. Therefore, a career as a vlogger has a lot to offer. If you want to know more about the Vlogger eligibility, roles and responsibilities then continue reading the article.
Publisher
For publishing books, newspapers, magazines and digital material, editorial and commercial strategies are set by publishers. Individuals in publishing career paths make choices about the markets their businesses will reach and the type of content that their audience will be served. Individuals in book publisher careers collaborate with editorial staff, designers, authors, and freelance contributors who develop and manage the creation of content.
Journalist
Careers in journalism are filled with excitement as well as responsibilities. One cannot afford to miss out on the details. As it is the small details that provide insights into a story. Depending on those insights a journalist goes about writing a news article. A journalism career can be stressful at times but if you are someone who is passionate about it then it is the right choice for you. If you want to know more about the media field and journalist career then continue reading this article.
Editor
Individuals in the editor career path is an unsung hero of the news industry who polishes the language of the news stories provided by stringers, reporters, copywriters and content writers and also news agencies. Individuals who opt for a career as an editor make it more persuasive, concise and clear for readers. In this article, we will discuss the details of the editor's career path such as how to become an editor in India, editor salary in India and editor skills and qualities.
Reporter
Individuals who opt for a career as a reporter may often be at work on national holidays and festivities. He or she pitches various story ideas and covers news stories in risky situations. Students can pursue a BMC (Bachelor of Mass Communication), B.M.M. (Bachelor of Mass Media), or MAJMC (MA in Journalism and Mass Communication) to become a reporter. While we sit at home reporters travel to locations to collect information that carries a news value.
Corporate Executive
Are you searching for a Corporate Executive job description? A Corporate Executive role comes with administrative duties. He or she provides support to the leadership of the organisation. A Corporate Executive fulfils the business purpose and ensures its financial stability. In this article, we are going to discuss how to become corporate executive.
Multimedia Specialist
A multimedia specialist is a media professional who creates, audio, videos, graphic image files, computer animations for multimedia applications. He or she is responsible for planning, producing, and maintaining websites and applications.
Welding Engineer
Welding Engineer Job Description: A Welding Engineer work involves managing welding projects and supervising welding teams. He or she is responsible for reviewing welding procedures, processes and documentation. A career as Welding Engineer involves conducting failure analyses and causes on welding issues.
Quality Controller
A quality controller plays a crucial role in an organisation. He or she is responsible for performing quality checks on manufactured products. He or she identifies the defects in a product and rejects the product.
A quality controller records detailed information about products with defects and sends it to the supervisor or plant manager to take necessary actions to improve the production process.
Product Manager
A Product Manager is a professional responsible for product planning and marketing. He or she manages the product throughout the Product Life Cycle, gathering and prioritising the product. A product manager job description includes defining the product vision and working closely with team members of other departments to deliver winning products.
QA Lead
A QA Lead is in charge of the QA Team. The role of QA Lead comes with the responsibility of assessing services and products in order to determine that he or she meets the quality standards. He or she develops, implements and manages test plans.
Structural Engineer
A Structural Engineer designs buildings, bridges, and other related structures. He or she analyzes the structures and makes sure the structures are strong enough to be used by the people. A career as a Structural Engineer requires working in the construction process. It comes under the civil engineering discipline. A Structure Engineer creates structural models with the help of computer-aided design software.
Process Development Engineer
The Process Development Engineers design, implement, manufacture, mine, and other production systems using technical knowledge and expertise in the industry. They use computer modeling software to test technologies and machinery. An individual who is opting career as Process Development Engineer is responsible for developing cost-effective and efficient processes. They also monitor the production process and ensure it functions smoothly and efficiently.
AWS Solution Architect
An AWS Solution Architect is someone who specializes in developing and implementing cloud computing systems. He or she has a good understanding of the various aspects of cloud computing and can confidently deploy and manage their systems. He or she troubleshoots the issues and evaluates the risk from the third party.
Azure Administrator
An Azure Administrator is a professional responsible for implementing, monitoring, and maintaining Azure Solutions. He or she manages cloud infrastructure service instances and various cloud servers as well as sets up public and private cloud systems.
Computer Programmer
Careers in computer programming primarily refer to the systematic act of writing code and moreover include wider computer science areas. The word 'programmer' or 'coder' has entered into practice with the growing number of newly self-taught tech enthusiasts. Computer programming careers involve the use of designs created by software developers and engineers and transforming them into commands that can be implemented by computers. These commands result in regular usage of social media sites, word-processing applications and browsers.
Product Manager
A Product Manager is a professional responsible for product planning and marketing. He or she manages the product throughout the Product Life Cycle, gathering and prioritising the product. A product manager job description includes defining the product vision and working closely with team members of other departments to deliver winning products.
Information Security Manager
Individuals in the information security manager career path involves in overseeing and controlling all aspects of computer security. The IT security manager job description includes planning and carrying out security measures to protect the business data and information from corruption, theft, unauthorised access, and deliberate attack
Automation Test Engineer
An Automation Test Engineer job involves executing automated test scripts. He or she identifies the project’s problems and troubleshoots them. The role involves documenting the defect using management tools. He or she works with the application team in order to resolve any issues arising during the testing process.
